For NEW posters. It’s been awhile. This one of t
Post# of 158771
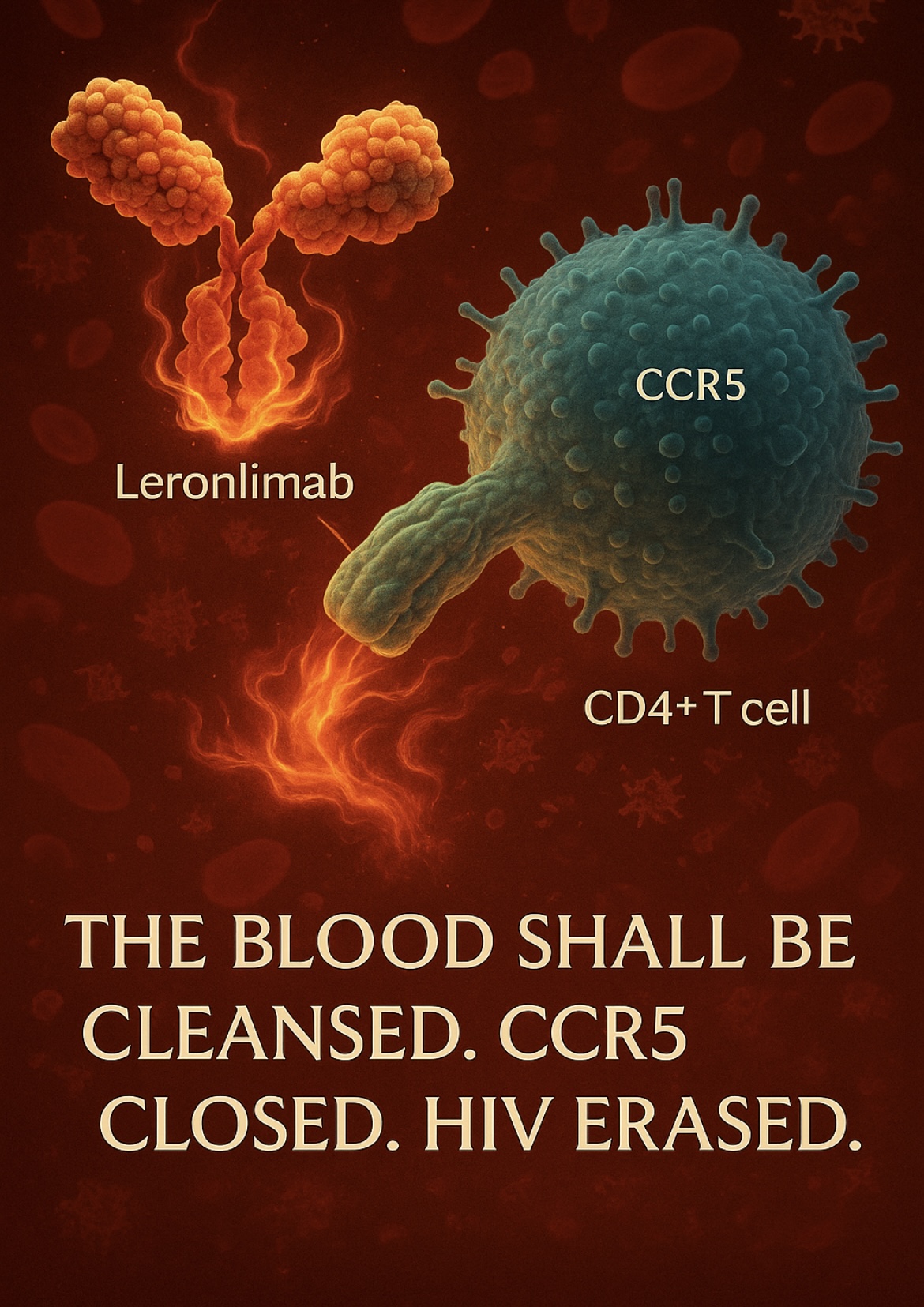
LERONLIMAB (PRO 140) is a humanized monoclonal antibody that targets CCR5 HIV‑1’s cellular entry point. By binding CCR5 with ultra-high affinity, it blocks the gateway for viral fusion and shuts down replication at its origin.
CLINICAL PROOF OF CONCEPT
• OPTIMIZE Trial (NCT02483078):
• Randomized, double-blind study in treatment‑experienced HIV patients
• 64% of Leronlimab patients hit ≥ 0.5 log₁₀ viral drop within just 1 week, vs. 23% on placebo (P = 0.0032).
• After 24 weeks, majority reached < 50 copies/mL, showing functional viral suppression.
MECHANISM & POTENTIAL
• Blocks CCR5–gp120 interaction, preventing R5‑tropic HIV entry into CD4⁺ T cells.
• Mimics natural CCR5Δ32 mutation, a known genetic resistance to HIV ().
• Once-weekly dosing due to >60-day receptor occupancy — far easier than daily ART regimens.
DEVELOPMENT STATUS & FORECAST
• Fast‑Track Designation from FDA for HIV treatment ().
• Phase IIb/III trial cleared from clinical hold Feb 2024; active enrollment expected in 2025.
• LATCH Trial (Leronlimab in Allogeneic Transplant to Cure HIV) launching mid‑2025 — direct move toward immunologic cure.
WHY THIS COULD ACTUALLY CURE HIV
1. It’s not just suppression it’s entry denial.
2. Reversible, manageable dosing vs. permanent gene editing.
3. Bridges current ART to real-world cure trials (e.g. LATCH).
4. Breast cancer and other trials highlight anti-inflammatory & anti-metastatic benefits.
ESTIMATED LATCH TIMELINE & IMPACT
• Mid-2025: LATCH begins at OHSU & UW targeting post-transplant viral eradication ().
• 2026–2027: Data maturation potential cure in case reports, expanded cohorts.
• 2028–2029: If successful, support BLA (Biologics License Application) for cure indication.
• 2025+: Companion monotherapy and HIV combo BLA underway repeated strong signals.
BOLD DECLARATION
“WE WILL CURE HIV.”
Not someday. Not someday but potentially in our generation.
The lock is built. The key is here.
LATCH is ready to test it on the world’s fiercest virus.
Took me seconds to write this one HOPE everyone has a wonderful weekend.
1. OPTIMIZE Trial (Phase IIb/III): pubmed.ncbi.nlm.nih.gov/39972543
2. Leronlimab Mechanism & CCR5 Binding: go.drugbank.com/drugs/DB05941
3. Leronlimab Fast‑Track & Details: en.wikipedia.org/wiki/Leronlimab
4. FDA Lifted Clinical Hold (Feb 2024): clinicaltrialsarena.com/news/fda-lifts-two-year-clinical-hold-on-cytodyns-hiv-trial
5. LATCH Trial Launch in 2025: cytodyn.com/newsroom/press-releases/detail/636/march-2025-letter-to-shareholders
 (7)
(7) (0)
(0)