Overlap, Leronlimab was given with haart the firs
Post# of 158034

Leronlimab was given with haart the first week, the they stopped haart
Quote:
Consenting patients will be shifted from combination antiretroviral regimen to weekly PRO 140 monotherapy for 48 weeks during the Treatment Phase with the one week overlap of existing retroviral regimen and PRO 140 at the beginning of the study treatment
They noticed two items. NP mentioned this since 2018.
1) Most failures happened in the first few weeks.
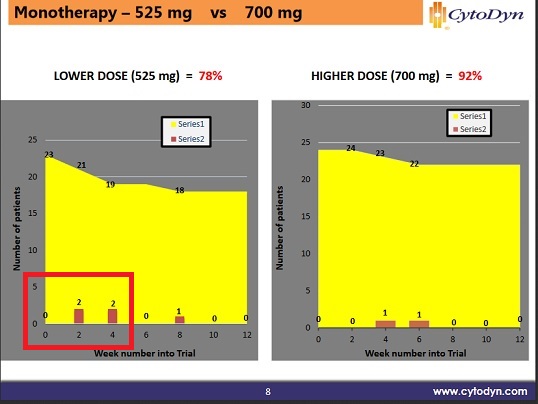
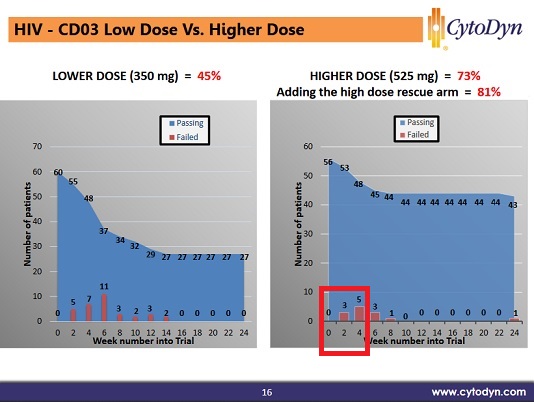
2) Once the patient reaches week 10, they usually continue indifferently. They posted both of these together for a while, then around March just posted the after 10 weeks results.
For #1 they found the half-life was wrong; they assumed it was 3.5 days based on a this 2010 paper from the trial using sc injections, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2856743/.
Here is my post talking about how they got the half-life wrong,
https://investorshangout.com/post/view?id=5429817
I found earlier studies that had two weeks or longer as the half-life, but the 2010 paper became common knowledge. That 3.5 day half-life is what is listed
here, https://aidsinfo.nih.gov/drugs/423/leronlimab/0/professional

Why it matters. It normally takes 5 half-lives to reach full dose, so counting failures at week 2 or 3 is before leronlimab reaches full dose, i.e. most of the failures was because of this issue.
So in Feb, when the enrolled the 525 vs 700mg patients, I believe they switched to a 3 week overlap before stopping hart. This will be used in the new mono study.
Also, with combo, the only used a one week when comparing the effect of leronlimab vs placabo. Well obviously, that wasn't no where near the full dose now knowing the longer half-life. IZ used a 2000mg loading dose, so the results without that with leronlimab are very impressive. And all the failures happened in the placabo group. Well the placabo group only had one week on leronlimab before failures could count, the other group had 100% success and had two weeks on leronlimab before failures could count. I think that plays a role, I emailed both of these to NP, and he seem interested, especially the loading dose instead of overlap, which the 2010 paper mentioned as a possibility.
Density
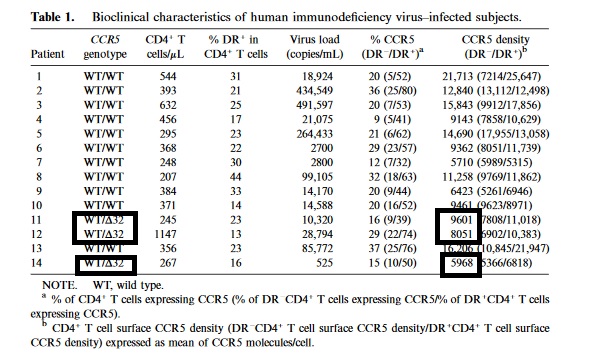
Different people have a different number of ccr5 receptors per cell. Delta 32 have zero, a delta 32 carrier has less than normal. Even without this it varies from person to person. They found the in phase 2 study, the people that failed had a higher density, which makes sense they have more receptors that need covered, so a higher dose would help cover all the receptors on the higher density group.
For density, my guess is the delta32 carrier just needs 350mg, between 5000 and 9000 ccr5 receptors per cell. Some people have twice that amount, but delta 32 doesn't predict it completely. Some have less without it.
https://academic.oup.com/jid/article/181/3/927/912958
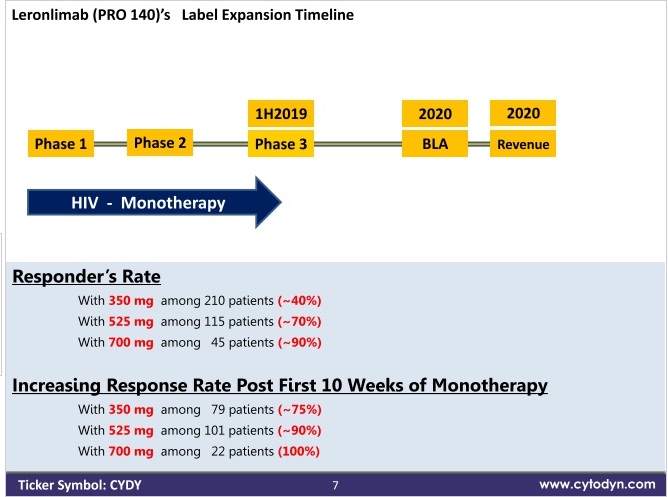
Now in the mono pivotal trial ind submission, they thought the first 10 weeks was critical, after that time it seems you can continue you dose. So they had everyone on 700mg the first 10 weeks and then switched randomly to 525 vs 700 after.
The FDA requested a density study. They believe as I do, at least I think that is why, is the density plays a big role here. The people failing the first 10 weeks are failing because their density is too high for the dose they were given. The overall efficacy goes up for 350mg to 500mg to 725mg supporting this. Also the phase 2 paper supports this idea, the failures had a higher density. Those people failed the first 10 weeks, then the people remaining had the correct density for the dose usually. Why 525mg shows a higher rate after 10 weeks, is it had more failures before 10 weeks weeding out the low density group. But 525mg has less overall efficacy that 700mg, to me it is just a statistical anomaly that 525 is better after 10 weeks because it had more failures before 10 weeks.
In fact there is a theory that hiv sparked exponentially after small pox vaccine stopped, the small pox used the same ccr5 receptor and there are studies that show after the vaccine the ccr5 receptor is downregulated, so it actually helped limit hiv spread. Once the vaccine was stopped being given in the 70s, it allowed hiv to spread rapidly, anyway that is the theory.
Anyway, the have a density test, I haven't seen the data, but they mono trial will probably assign dose based on density and the results could be good or better than after the 10 week data for the 350mg and 525mg dose. For the 700mg dose, improvement I feel depends on if they will have an upper limit on density, meaning some people can't participate because their density is too high (i.e you don't want to push all the extremely high density group up to 700mg, with that dose still not enough to cover) I don't know if that is a possibility, just guessing it might be, but Dr. P has done the study and the FDA is reviewing it and they will decide the protocol based on the results.
 (6)
(6) (0)
(0)