Here is a table containing the chemo drugs used wi
Post# of 158025

Quote:
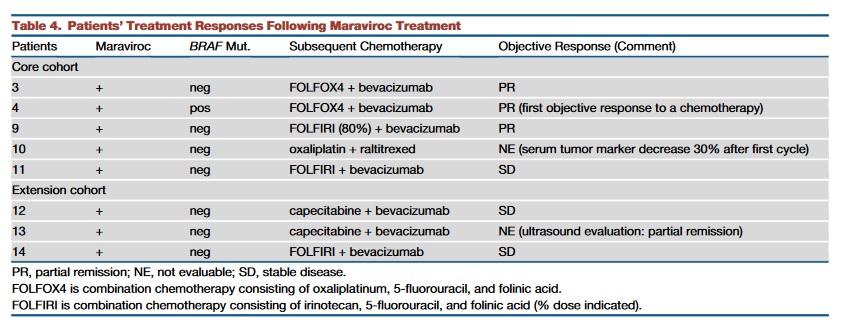
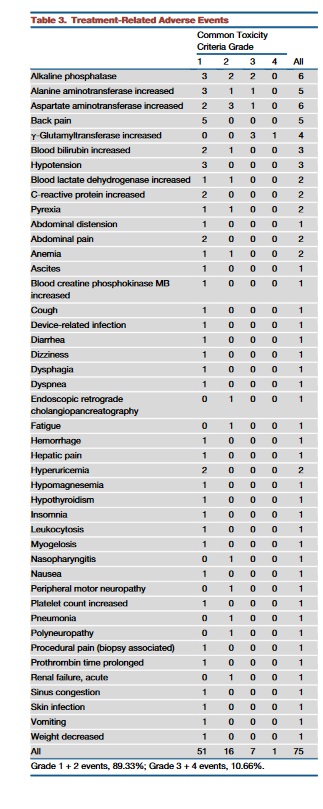
All patients had received previously all of the standard-of-care treatments (including regorafenib for some patients,Tables 1 and 2) with an average of 4.3 lines of previous treatment. Nevertheless, maraviroc treatment was very well tolerated. The most commonly observed treatment-related adverse events were mild elevation of liver enzymes (Table 3). Median overall survival was 5.06 months for the core cohort (95% confidence interval, 3.06 months to infinity). From the 11 patients of the core cohort, five decided to be re-exposed to chemotherapy after participation in the trial. Patients were allowed to receive maraviroc in combination with chemotherapy, as the biopsies from the trial had shown anti-tumoral effects at the tissue level. Of these five patients, three had objective partial responses (60%,Figures 5F and 5G), one patient had stable disease, and one was not evaluable, so that a tumor control rate of 80% was achieved(Table 4).
Quote:
Looking at the trial data, it is nevertheless clear that the subsequent objective treatment responses are very encouraging. Historically the objective response rates in patients on or after the third line of chemotherapy are around 5%–10% (Nielsen et al., 2014). No large datasets exist on patients on or after fourth-line therapy, but objective responses were generally not observed and chemotherapy is only applied for symptom control(Arkenau et al., 2009). This is in contrast to our result of a high response rate in this advanced situation.
Quote:
The MARACON-001 phase I trial (‘‘Treatment of Advanced Colorectal Cancer Patients with Hepatic Liver Metastases using the CCR5-Antagonist Maraviroc’’, clinical trials.gov identifier NCT01736813, EudraCT 2012-000861-18) involves daily exposure to maraviroc (300 mg twice daily), a highly selective CCR5 inhibitor, for 2 months. Safety and feasibility are the primary endpoints of this trial, being conducted according to the Declaration of Helsinki and relevant International Conference on Harmonization Good Clinical Practice guidelines, and with approval from the ethics committee of the University of Heidelberg. All patients had received all current standard-of-care treatment options and were now refractory to standard chemotherapy
 (4)
(4) (0)
(0)