Prurisol efficacy signal (Phase 2a, 200 mg) ht
Post# of 72451

Quote:
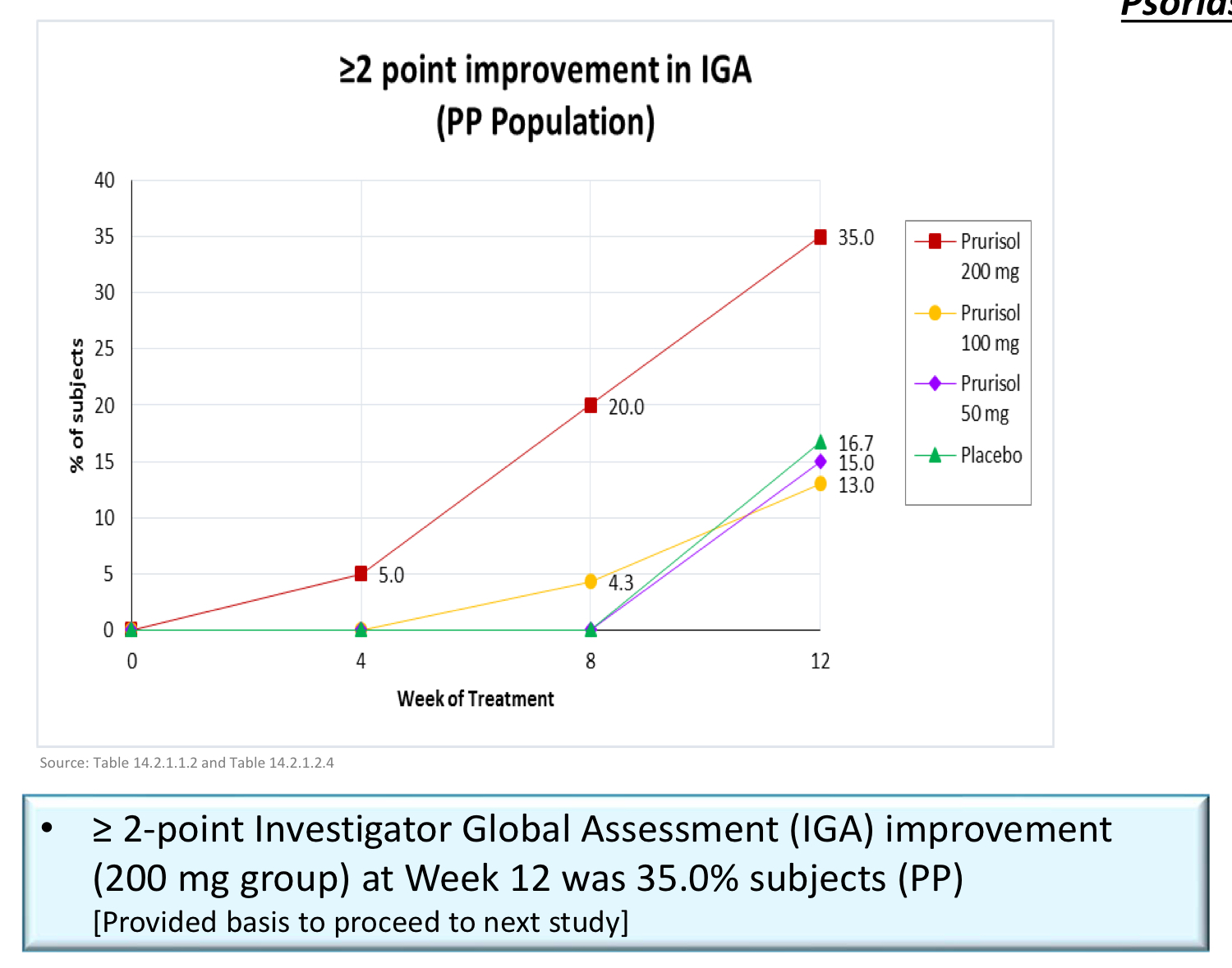
Sub-population analyses further showed greater efficacy demonstrated in patients who had a baseline IGA score of 3 (“moderate”) as compared to those with a baseline score of 2 (“mild”). Some of these patients even experienced a 3-point reduction in their IGA score, going from “moderate” to “clear.” This suggests Prurisol may be more effective in treating moderate to severe psoriasis patients to a greater degree than those patients who exhibit less severe symptoms. In moderate to severe psoriasis studies, the placebo response also tends to be lower.
http://www.ipharminc.com/press-release/2016/1...y-endpoint
Quote:
Among patients participating in the study with the severest form of psoriasis, those having a baseline IGA score of 3 (“moderate”), the primary endpoint was met in 46.2% of patients who received Prurisol 200mg. This data was derived from analyses of all patients randomized across all 9 participating study sites.
Additional preliminary data analyses of secondary endpoints show patients who received any dose of Prurisol, regardless of the treatment arm, had a 1-point improvement (using the IGA scoring system) at a higher rate than that of patients in the placebo arm. This is another clear indication of the drug’s efficacy.
http://www.ipharminc.com/press-release/2016/1...-psoriasis
Quote:
The data from the first Phase 2 trial is very impressive and clearly showed a stronger therapeutic benefit at the 200mg dosing level in patients wiith moderate psoriasis, as measured by the Investigator’s Global Assessment scale. I am very excited about the results that already suggest benefit starts at 2 weeks and further improves by the end of the study at 12 weeks. Let’s say that this gave us a great 15,000-foot view of the Prurisol activity. This is what an initial Phase 2 trial is for. The data certainly point to an even stronger clinical benefit at higher doses in patients with more severe disease. The purpose of the Phase 2b trial in patients with moderate to severe psoriasis is to better define dosing, examine responses using endpoint(s) that will be used in Phase 3 pivotal trials and to gain further experience with safety.
https://seekingalpha.com/article/3988240-inte...-bertolino
The psoriasis xenografted SCID mouse model used for Prurisol is a reliable indicator of clinical efficacy. Etanercept (Enbrel), the psoriasis drug with +$1B in sales, used the same preclinical model. Here’s the abstract of the study.
Translating clinical activity and gene expression signatures of etanercept and ciclosporin to the psoriasis xenograft SCID mouse model.
Quote:
BACKGROUND: The psoriasis xenograft severe combined immunodeficiency (SCID) mouse model is used in drug discovery to obtain preclinical proof-of-principle of new antipsoriatic drug candidates. Validation of this model by antipsoriatic therapeutic agents in clinical use is important to understand its utility as well as its limitations. The effects of the clinically efficacious antitumour necrosis factor-a biologics have not yet been demonstrated in the psoriasis xenograft SCID mouse model.
OBJECTIVES: To investigate the effect of etanercept and to explore the time-dependent changes induced by ciclosporin on psoriatic biomarkers at the gene expression level in the psoriasis xenograft SCID mouse model.
METHODS: Xenografted SCID mice were treated either with etanercept and vehicle for 2 weeks or with ciclosporin and vehicle for 2 and 4 weeks, respectively. Treatment-induced changes in the psoriatic grafts were assessed by gene expression analysis and compared with published clinical microarray data. The grafts were further evaluated by histology and immunohistochemistry.
RESULTS: Etanercept induced normalization of gene expression, which correlated with a significant reduction in epidermal thickness as well as a decrease in the number of proliferative cells. Anti-inflammatory activity induced by ciclosporin preceded the reduction in epidermal hyperplasia. Comparison of the etanercept- and ciclosporin-induced gene expression signatures with clinical microarray data showed significant correlations.
CONCLUSIONS: Efficacy of etanercept and ciclosporin could be translated to the psoriasis xenograft SCID mouse model.
https://www.ncbi.nlm.nih.gov/pubmed/22050597
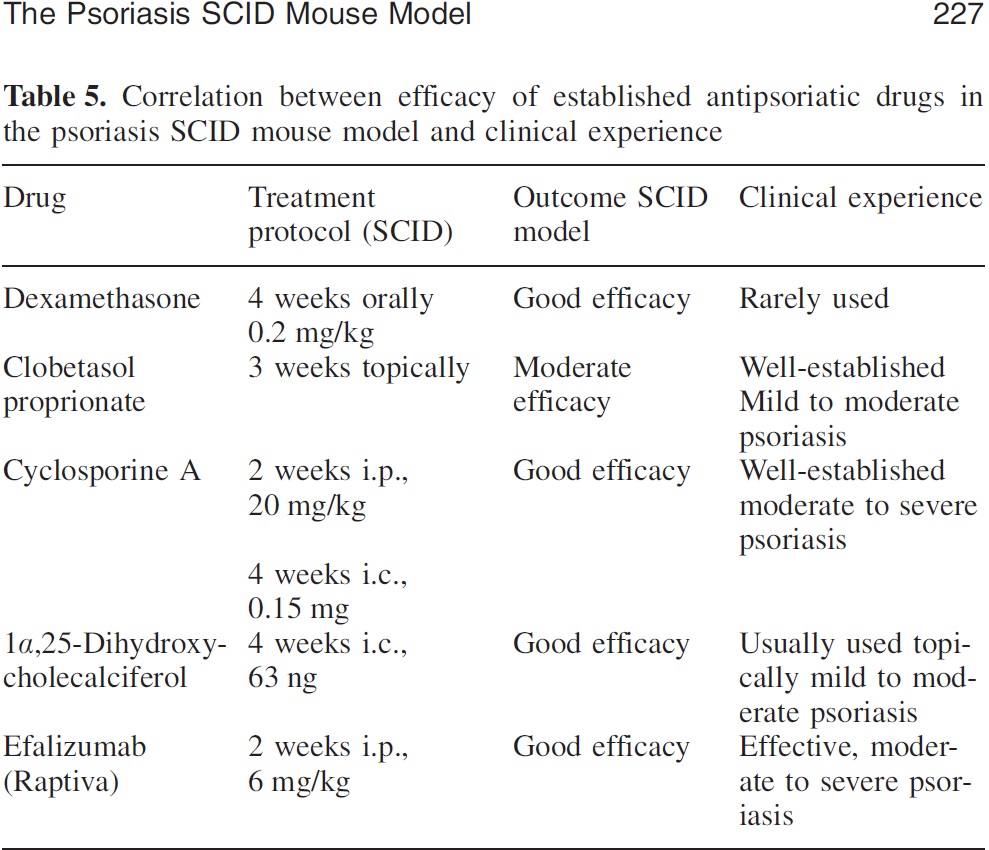
This table shows the correlation between preclinical model and clinical results.
 (0)
(0) (0)
(0)Innovation Pharmaceuticals Inc (IPIX) Stock Research Links
