(Total Views: 736)
Posted On: 02/22/2022 4:17:21 AM
Post# of 157976
The Pfizer trial is using the National Institute of Allergy and Infectious Diseases (NIAID) ordinal scale shown below. The second image is the WHO scale. NIAID doesn't have a breakout for moderate patients WHO breaks out moderates as either hospitalized no oxygen or hospitalized with low flow oxygen. Which scale leronlimab is using is unknown. It doesn't match up with NIAID.
As you can see Pfizer even excludes the high moderates (score 5) on the WHO scale. Leronlimab's inclusion/exclusion criteria match up with 5 or 6 on the WHO scale. So completely different patient parameters. For supplemental oxygen at any point it's irrelevant to the Pfizer trial enrollment because any patients with supplemental oxygen are excluded if they are on it from the first day of their potential drug use.
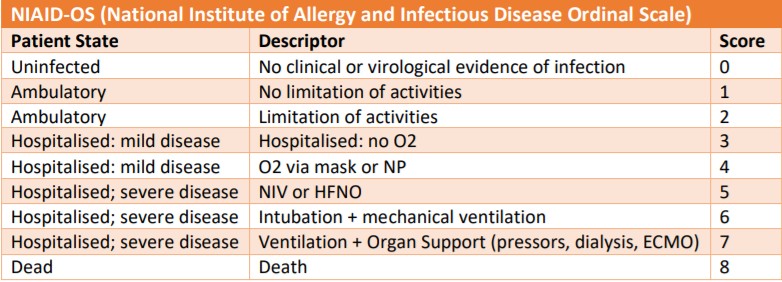
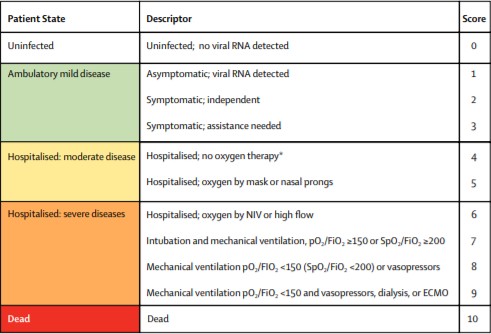
As you can see Pfizer even excludes the high moderates (score 5) on the WHO scale. Leronlimab's inclusion/exclusion criteria match up with 5 or 6 on the WHO scale. So completely different patient parameters. For supplemental oxygen at any point it's irrelevant to the Pfizer trial enrollment because any patients with supplemental oxygen are excluded if they are on it from the first day of their potential drug use.


