Univec CEO David Dalton they say is a rock star, l
Post# of 6857
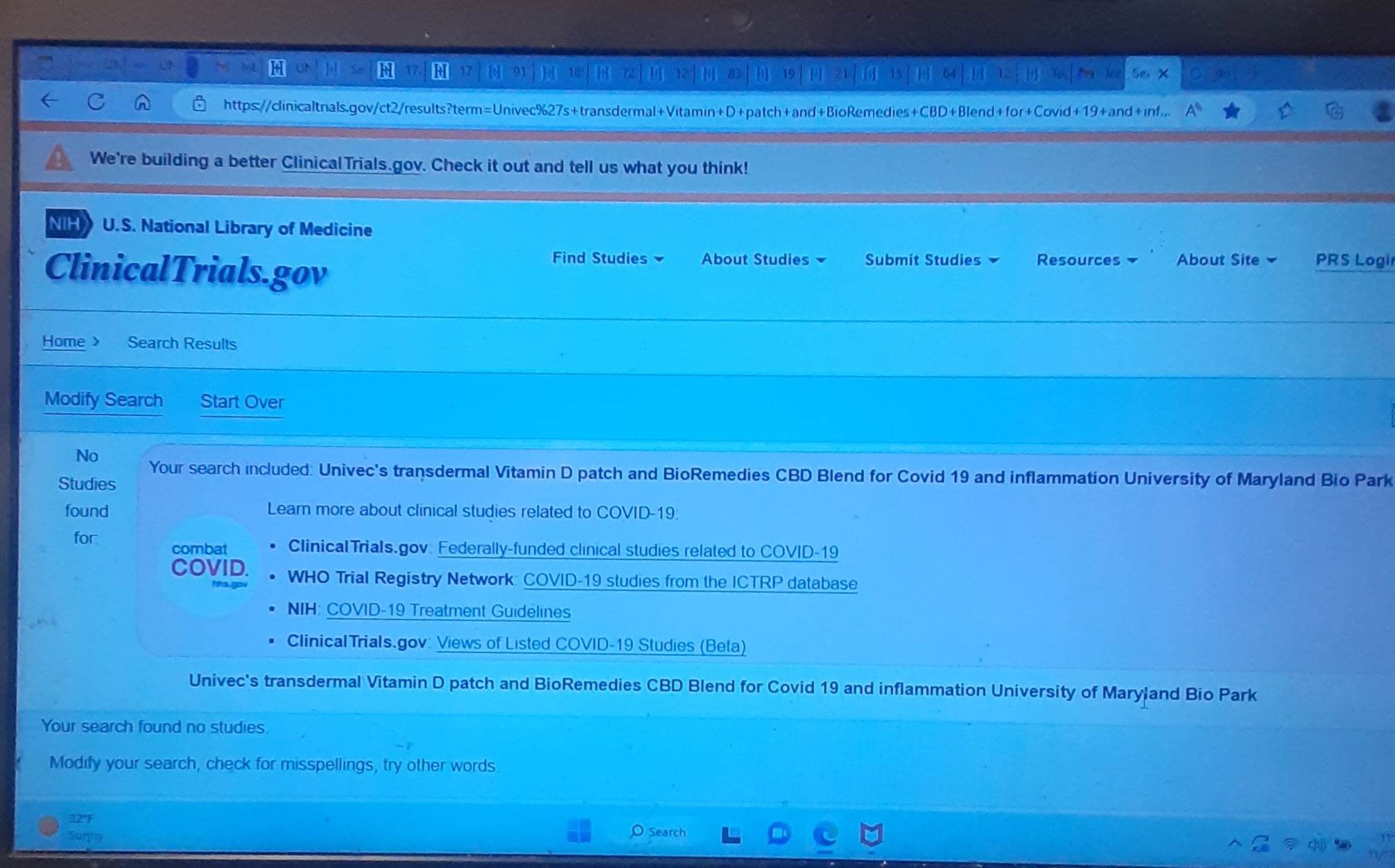
So all believing the speculative/hype about Univec having FDA approved products its not happening.
We were told that they had transdermal patches in trials for OPIOID Addiction and had made it through 2 stages (I know I talked with them and reported back about the progress) Plus these below
![171060251_qnzxp455449411_Tweet![1][1].png](https://investorshangout.com/images/MYImages/171060251_qnzxp455449411_Tweet![1][1].png)
B/R an Univec were conducting PRE-CLINICAL TRIALS with University of Maryland School of Medicine, we were mis-lead by Dalton and B/R about these patches, they told me they were for Pharmacies only which was SPECULATED BY MANY they would more likely have FDA approval.
Long story short they aren't even being sold anywhere or did they make it any further in the trials NOR WERE THERE ANY STUDIES/TRIALS FOR A TRANSDERMAL OPIOD ADDICTION PATCH

https://clinicaltrials.gov/ct2/results?term=U...rch=Search
Do clinical trials require FDA approval?
To certify compliance with ClinicalTrials.gov requirements, FDA requires that applicants complete and submit Form FDA 3674 with certain human drug, biological product, and device applications and submissions.Mar 7, 2022
https://bioremediesmd.com/products/
 (3)
(3) (0)
(0)