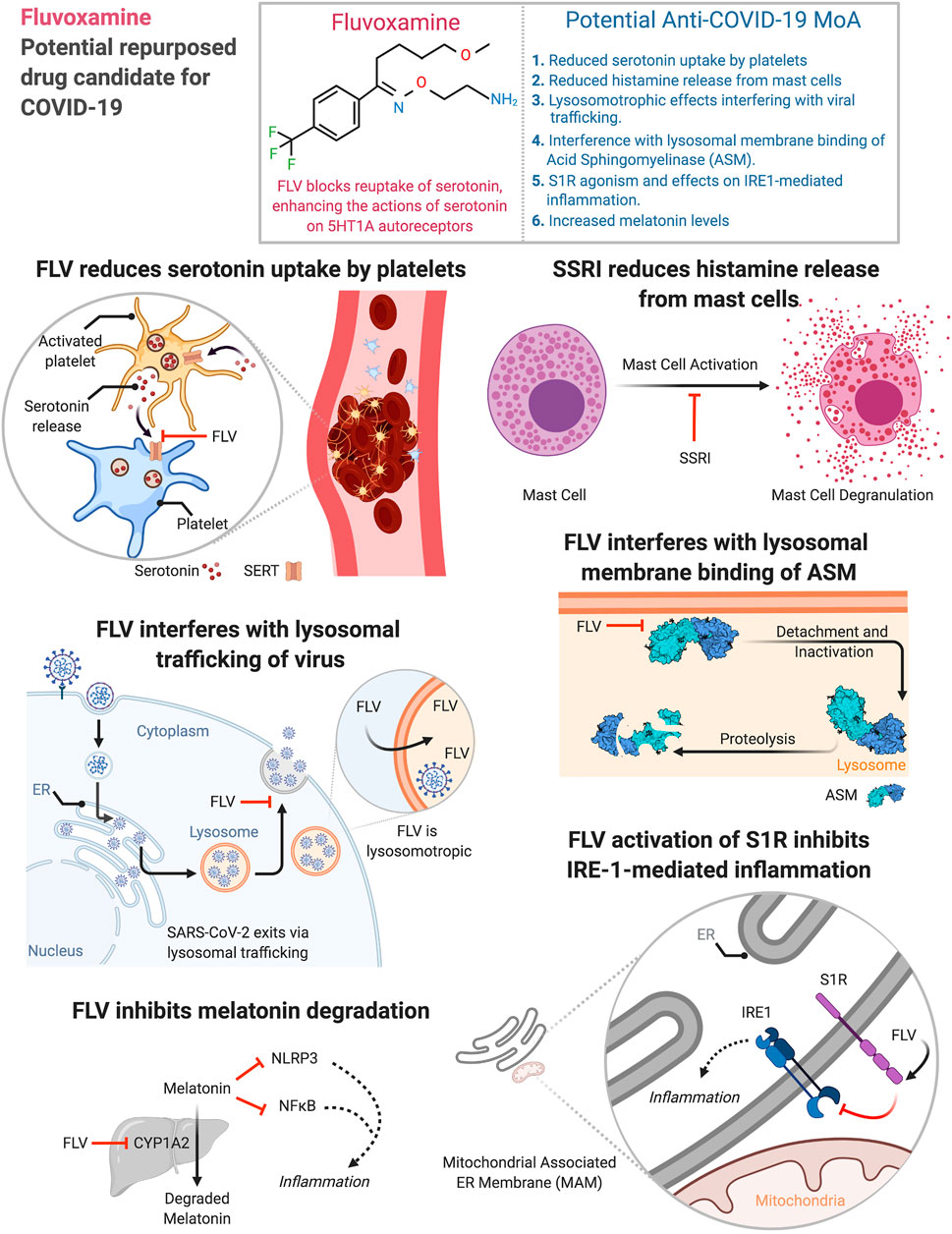
Mechanism of SSRI/other anti-depressant use for tr
Post# of 158215
https://www.nature.com/articles/s41380-021-01021-4
Antidepressants could be antiinflammatory, and could prevent infection of endothelial cells.
There was one smaller randomized controlled trials of fluvoxamine before the larger (N=1500+) one in Brazil.
Also one small real world "trial" showing excellent results (0/65 hospitalized on fluvoxamine vs. 4/48 who declined the drug).
https://www.wsj.com/articles/is-fluvoxamine-t...1640726605
https://www.frontiersin.org/articles/10.3389/...52688/full
shows perhaps it is the Sigma1 receptor agonism that makes it potentially useful as an antiinflammatory drug. Anavex (AVXL) is having some early success with its S1R agonists.
It's hard to argue with the Brazil trial results:
https://www.thelancet.com/journals/langlo/art...4/fulltext
Quote:.
There was one death in the fluvoxamine group and 12 in the placebo group for the per-protocol population (OR 0·09; 95% CI 0·01–0·47)
But I guess people argue ITT versus PP all the time.
Quote:
Our primary outcome was a composite endpoint of medical admission to a hospital setting due to COVID-19-related illness defined as COVID-19 emergency setting visits with participants remaining under observation for more than 6 h or referral to further hospitalisation due to the progression of COVID-19 within 28 days of randomisation. Because many patients who would ordinarily have been hospitalised were prevented from admission due to hospital over-capacity during peak waves, the composite endpoint addresses both hospitalisation and a proxy for hospitalisation, retention in a COVID-19 emergency hospital setting. This region of Brazil implemented hospital-like services in the emergency settings with 50–80 bed units providing services including multiday stays, oxygenation, and mechanical ventilation.
It's hard to run a trial during a pandemic! I think a cheap and safe $4 drug is well worth it, even if the results are only somehow half as good as reported, and the "hospitalization" endpoint used imperfect.
It's interesting the DSMB stopped the study early and said it's unethical to continue randomization since the benefit was so overwhelmingly positive, yet there remain critics and NIH hasn't adopted it as a recommendation. I guess they should have kept randomizing more people to satisfy the critics and skeptics?
I hope our LL trials in Brazil are judged to be cleaner by our PI who criticized the FLV trials done there. I can't imagine he would say anything bad about a small study he is running that just became smaller. Hopefully it fills soon and we get outstanding data in critical patients from Brazil.
 (7)
(7) (0)
(0)