Have received some inquiries regarding the Power w
Post# of 157005

As we know we had only 62 patients in this group and got an overall p-value of 0.005.
If we assume once more mean of 38.5 days for placebo with an overall SD of +-10 we obtain that, the achieved power of our "trial" (for O-2 Critical patients) was app. 58.4 % (due to the reduced number of patients, 62).
FDA normally requires 80%-90% for approval but I assure you it has issued EUA for results with much less indication of efficacy.
Leronlimab's is all over the place, sure is "only" 62 patients, sure, it helps the mostly critical patients, but .... every single measurement showed some improvement ...
Look at the charts below ... every single one of them ....
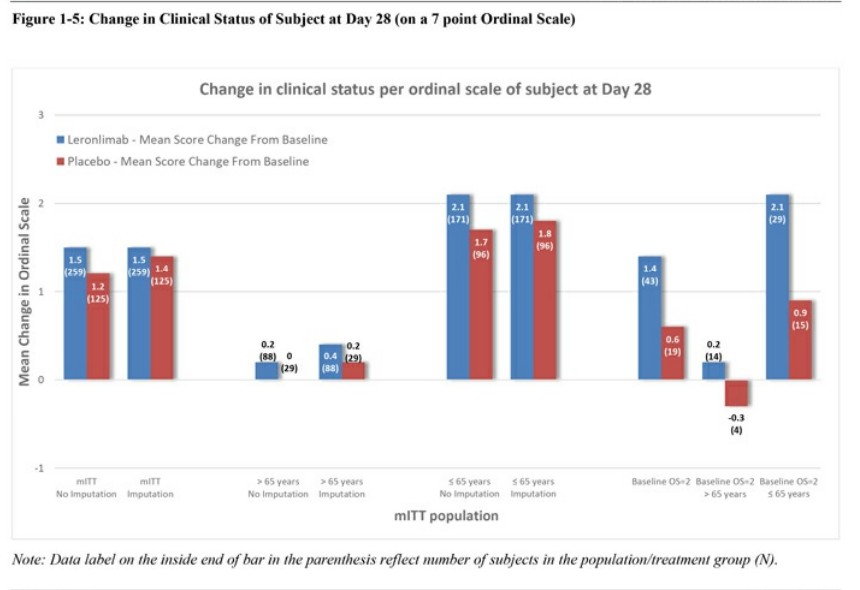
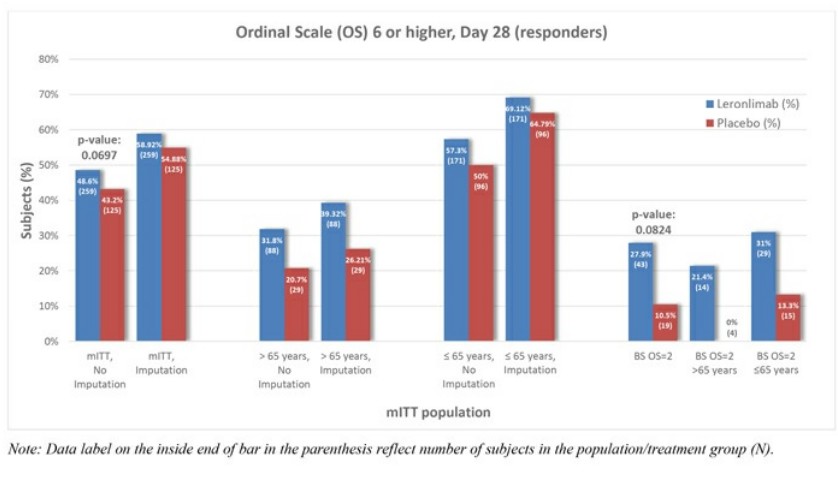
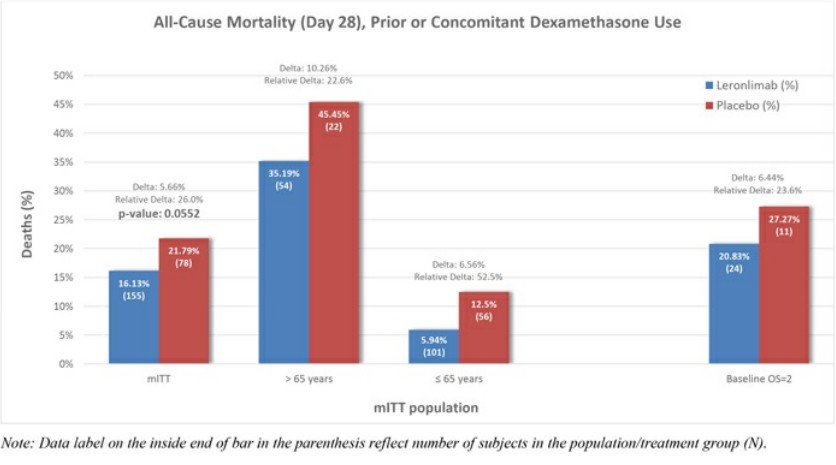
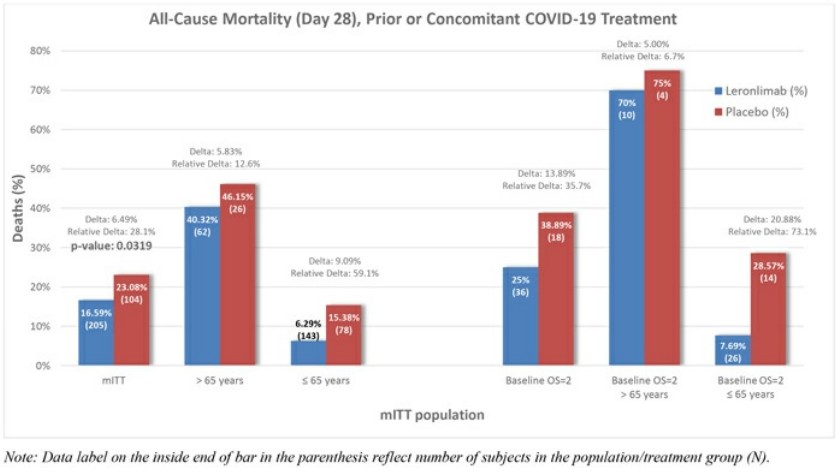
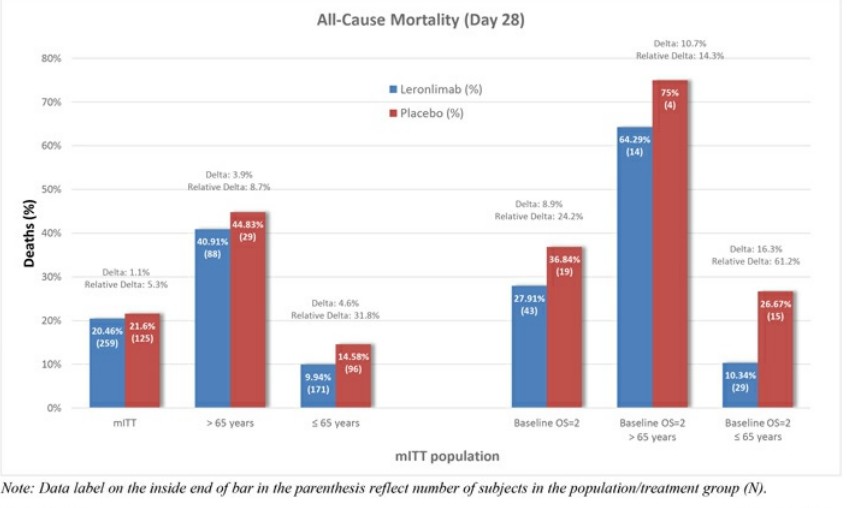
Question: if a drug is demonstrably SAFE and it improves COVID patients, why not allow it ???
I don't have a good answer .... not after witnessing what "they" did with other companies whose names I don't want to mention
 (29)
(29) (0)
(0)