As we get closer to our “day in court” it is a
Post# of 155271

There have been several discussions about NP and the BOD. There is some truth in all of them (positive or negative). I try to focus on the facts and there is one evident right now: they have brought us to to the point in which the benefit (or lack of) Vyrologix in COVID-19 will be ascertained.
As we approach the interim analysis for 293 patients what are the numbers we need to “hit” for likely approval??
We discussed at length before that we needed to have a p-value of less than 0.05 in the first readout. This, assuming that the DSMC uses the O’Brien-Fleming boundary points for statistical significance. The table below lists the values for different interim frequencies.
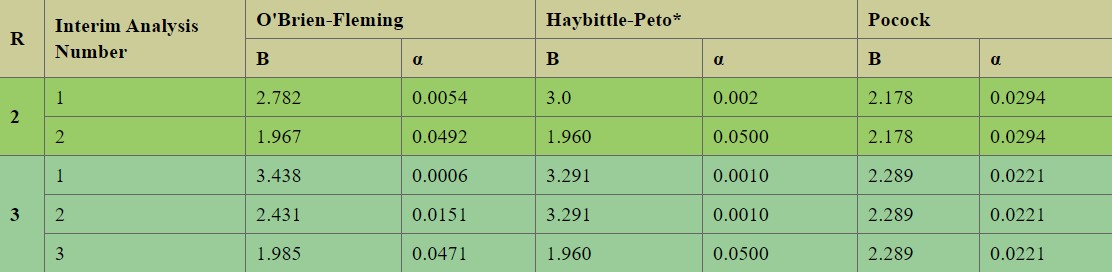
At the time, as we did not know that there will be a recommendation for yet another interim we assumed the required 0.05 (actually 0.054) p-value. Now, as we know there are three looks at the data, this number should had been 0.0006 (big difference).
Of course, this numbers are for "planned" analysis and that is the advantage of not un-blinding the data. In any case, we are now in the point where our interim will need a p-value of less than 0.0151 (assuming O’Brian criteria). This could be a bit higher as we will be at 75% (rather than 66% of patients) at time of analysis.
Please note that the Pocock method it provides the best possibility of early trial termination, however is the most difficult to comply with at the end of the trial. The Haybitte-Peto provides intuitive boundaries constant in the intermediate readouts and equal to the initial (defined) significance level at the end.
To summarize, we are likely looking at around 0.0151 for our next interim and at 0.0471 for our final readout in case we complete our 90 patients full enrollment (meaning a 0.0029 overall penalty).
Another point that will need to be taken into consideration id the power at the time of the calculations. It is a positive that the board considered that at 293 this could be met as well.
The problem with giving numbers is that one is stating truths. In this case, we do not know even which criteria (methodology) is being used by DSMC/FDA so please take the above as a guideline only .
As far as I am concerned a reduction of deaths of, say, 30% (producing a p-value greater than 0.0151), for me, should produce a swift authorization from FDA. And I am not speaking as an investors but as a human being.
As we speak more than 250000 have died in this country alone. Enough is enough.
 (20)
(20) (1)
(1)