As some of you have already stated before, and I a
Post# of 157832

Some have asked about the maximum p-value to gain approval at interim. Well, no hard lines here, but some advocate for a value of 0.005. This number is not magic, however one consideration that is often forgotten is the Power of the trial.
Power is the probability of identifying a real difference between the SOC and LL with the statistical test. Or put it in another way: is the probability that we will detect a predetermined difference (p-value) between SOC and LL, if it truly exists.
Imo, we were not approved right away because our power was still not there (our p-value being very good). Please bear in mind that this is proportional to the number of patients. This is why BP enrolls thousands of patients in their trials.
Please refer to the table below. I made some Post-hoc calculations of Power for a binomial endpoint to illustrate this (numbers are clearer).
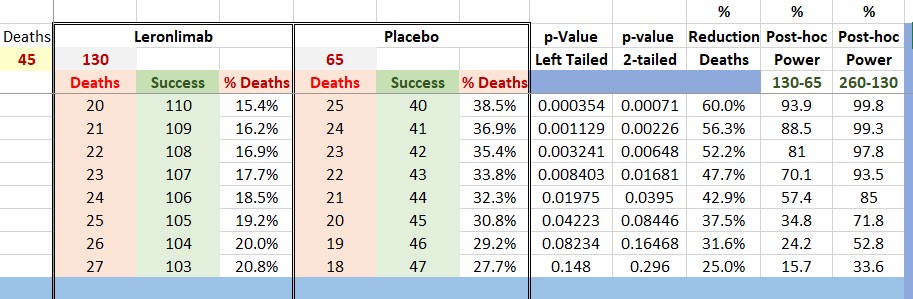
As you can see, with 195 patients to achieve a power of 81% our p-value should be 0.00648. If we had a p-value of 0.01681 (at interim with 45 deaths) this would be only 70.1% !!!. FDA normally uses either 80% or 90%.
The same distribution of deaths (in %) would produce a power of 93.5% for 390 patients.
Please note that the same distribution of deaths would not produce the same p-value for 390 patients, we are just comparing the power of the trial.
The moral here is that, very likely, we have good results but the Board wants to increase the trial power by augmenting the number of patients at analysis. Now, it is extremely encouraging to see that the statistician decided to have another peek at 75% enrollment rather than full as this means that we were close to target, so to speak.
If you ask me, I think our trial is a bit underpowered the way it is (with 390 patients only), so this is very encouraging results.
 (16)
(16) (0)
(0)