I have been an investor in CYDY for few years now.
Post# of 158237

Having said that, now to the communication: the key here is the biostatistician’s opinion (we know we will no be stopped due to safety issues.). In order to eventually succeed we need to have good results in our interim . A bad ratio of deaths (LL/SOC) in this will produce a result that will mean that even continuing the trial with more patients will not produce a statistically-meaningful outcome. Or, put it in other words: LL helps somewhat but is not good enough to meet statistical significance. Or, put yet in another way: Leronlimab is not saving enough lives for the criteria normally used to approve.
A continuation of the trial means we are in our way to reach significance.
So, continuation of the trial is good news. Don’t take me wrong: of course, I would better have the trial ended right now (meaning the reduction of deaths, ROD henceforth, was in the order of 50%, p-value around 0.005). So, let’s assume this did not happen (don't know yet what will be communicated next Tuesday), let’s say we achieved ROD of 32.8% (the famous S. Hahn’s number that, implies a p-value of 0.068 close to statistical significance). This is an extremely good result !!! (we are saving 33 lives out of 100).
Yet, we might be requested to continue the trial as the power (with 195 patients) is not good enough (this is worse with a 2:1 allocation ratio requiring 12% more patients than a trial using 1:1 to detect the same size effect with equivalent power). All we have to do is to complete the trial and, hopefully, continue in the same trend of results.
Now, let’s assume our interim ROD was 20%, some would argue that this is still very good (we are saving lives !!), the problem here is that continuing the trial will unlikely result, at the end, in a p-value of <0.05.
Once again, continuation of the trial means it is understood that we got first-rate results and can meet the endpoints at the end. Clinical trials are difficult events (just check how many are successful overall). Being good does not mean one gets approved.
Another angle is the Severe/Critical split. The number of deaths stands at 23%. Most of them are coming from Critical patients, of course, as the overall patient averages are lower. What if the statistician determines that Leronlmab is extremely effective with the latter ??? The recommendation would be to continue with only Critical patients and/or increase the overall number to achieve a well-powered cohort for each group. Remember, we are running and adaptive trial.
Yet, there is also the secondary end-points which will add credence to the thesis of efficacy.
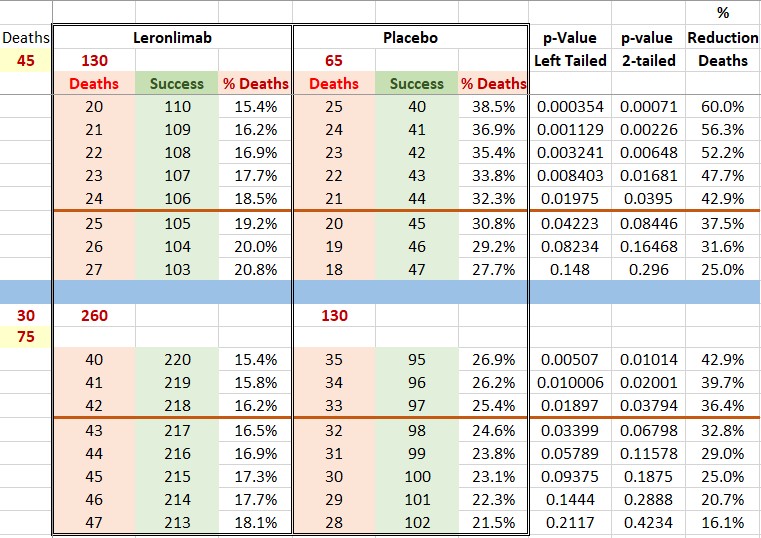
Please find below a table with the interim numbers and a scenario where in the second part of enrollment 30 patients die (due to better care as compared with first half) for a total of 75 deaths, and what it means at the end in respect to p-values and RODs. Right now (prior to interim) to "hit the ball out of the park" we need around 50%, at the end of the trial we will need around 33%. However, given the pandemic severity and the lack of alternatives I would not be surprised if even a ROD of 20%-25% is accepted and we get approval. I am using a two-binomial distribution test for the p-value calculations. The red lines are levels at which I recon we will be approved for interim and end of trial respectively.
Is a waiting game. Unfortunately we will not get any numbers, just opinions from the DSMB, mostly the biostatistician who will advise on what to do next.
And, we will depend on what CYDY wants to tell us as they do not have to give us the whole enchilada. Hopefully we will get it and some, however, bear in mind that there might not be enough data to keep us happy as the DSMB is obliged to recommend and not to report data.
I remain very long and very optimistic about our prospects
 (19)
(19) (0)
(0)