Two PRs from last year about ROT, and post I did l
Post# of 157857

https://www.cytodyn.com/newsroom/press-releas...-occupancy
CytoDyn Announces Major Discovery for its Monotherapy Indication
Download as PDF
June 18, 2019 6:00am EDT
CytoDyn and IncellDx Report the Successful Development and Validation of an Assay to Assess the Responders for Monotherapy HIV Patients
This assay could predict in 2 hours the responder’s rate for a monotherapy patient candidate and the appropriate dose for each patient at the time of screening
VANCOUVER, Washington, June 18, 2019 (GLOBE NEWSWIRE) -- CytoDyn Inc. (OTC.QB: CYDY), (“CytoDyn” or the “Company”), a late-stage biotechnology company developing leronlimab (PRO 140), a CCR5 antagonist with the potential for multiple therapeutic indications, announced today significant progress in securing the therapeutic effect and safety of leronlimab (PRO 140) with the development of an assay that can reliably determine a patient’s CCR5 status. Currently, all new patients in the Company’s monotherapy trial are being evaluated with this newly developed screening-level test with the objective of identifying patients expected to respond to a monotherapy versus those not expected to respond due to CCR5 density.
“The development and implementation of an assay that can rapidly and accurately identify potential responders to leronlimab offers a tremendous technical advancement with the potential to more precisely target leronlimab on patients most likely to respond to treatment,” stated Dr. Nader Pourhassan, President and CEO of CytoDyn. “We are excited about our partnership with IncellDx to provide the technical support to explore the power and potential of CCR5 inhibition,” continued Dr. Pourhassan. “With over 110 HIV active patients on leronlimab monotherapy for almost one year and five patients from our original Phase 2 monotherapy extension now reaching almost five years on monotherapy without viral breakthroughs, we are very excited with our collaboration with IncellDx and Bruce Patterson, M.D., former Medical Director of Virology at Stanford University Hospitals and Clinics. With Dr. Patterson’s expertise, we are confident that we will be able to much more efficiently identify responders in HIV, and potentially in many other indications, including our ongoing cancer research.” Dr. Pourhassan concluded, “The continued clinical success of leronlimab in HIV underpins our soon to begin partnership with Thai Red Cross AIDS Research Centre for the PrEP clinical trial that will exclusively utilize leronlimab as a monotherapy.”
“The HIV-co-receptor CCR5 has both genotypic differences in patients, as well as being a highly regulated receptor with different amounts expressed on different cells under certain conditions such as inflammation. The development of a portfolio of assays, including CCR5 genotyping and the assay being reported today that reliably and timely identifies a patient’s CCR5 status, offers a leap in the understanding of potential response to treatment with leronlimab,” stated Dr. Bruce Patterson, CEO and Founder of IncellDx. “I am excited to continue the very positive and effective partnership with CytoDyn to potentially help bring leronlimab to the right patients at the right time, in furtherance of the clear opportunity afforded by personalized medicine,” concluded Dr. Patterson.
https://www.cytodyn.com/newsroom/press-releas...-occupancy
CytoDyn Finalizes Development of Receptor Occupancy Test to Guide Pivotal Monotherapy Discussions With the FDA
VANCOUVER, Washington, July 25, 2019 (GLOBE NEWSWIRE) -- CytoDyn Inc. (OTC.QB: CYDY), (“CytoDyn” or the “Company”), a late stage biotechnology company developing leronlimab (PRO 140), a CCR5 antagonist with the potential for multiple therapeutic indications, today announced the completion of development of the Receptor Occupancy Test to measure the expression of CCR5 in HIV and tumor cells that are occupied by leronlimab. Development of this test could more precisely guide CytoDyn in identification of patients at screening for monotherapy.
To date, over 830 HIV patients have participated in clinical trials with leronlimab (PRO140), including over 600 patients in ongoing and previous monotherapy trials. There have been no drug related serious adverse events (“SAEs”) reported in any of the HIV patients treated with leronlimab. In addition, the response rate for the first 10 weeks of leronlimab monotherapy in HIV patients has approximated 95% with the 525 mg dosage. Identifying the patients who can respond to leronlimab monotherapy at the screening is critical for therapeutic success. As such, the development of the Receptor Occupancy Test is a significant milestone that may further increase the monotherapy response rate.
“The development of the Receptor Occupancy Test is an important milestone for CytoDyn because it may allow more precise screening for leronlimab monotherapy,” stated CytoDyn President and CEO, Nader Pourhassan, Ph.D. “Over 600 HIV patients that have taken leronlimab as a single agent over the years have had no drug related SAEs,” continued Dr. Pourhassan. “The potential for leronlimab to be a safe and effective treatment for HIV with highly accurate dosing is an important driver of continued excitement and support from key opinion leaders within the medical community,” Dr. Pourhassan concluded.
Significantly, CytoDyn has been granted an in-person meeting with the U.S. FDA to discuss its pivotal monotherapy protocol that could lead to label expansion approval should leronlimab have its first approval as a combination therapy. To date, 110 patients have reached approximately one year of monotherapy trial with six patients in the extended portion reaching almost three years. Four patients in the extension arm of a previously reported Phase 2 monotherapy trial have continued on leronlimab monotherapy for nearly five years with no drug related SAEs and no viral breakthroughs.
The Company will host a conference call for investors and media on Tuesday, July 30, 2019, to discuss the latest developments, provide a comprehensive update about CytoDyn, along with comments from Drs. Sacha, Patterson and Lalezari.
https://investorshangout.com/post/view?id=5565423
Quote:
For density, my guess is the delta32 carrier just needs 350mg.
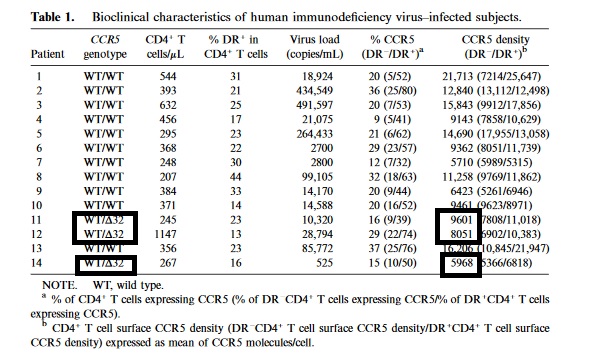
https://academic.oup.com/jid/article/181/3/927/912958
 (4)
(4) (0)
(0)