Yeah, that is so far leronlimab's strong suit. BMS is the farthest along with cancer, they have 5 cancer trials ongoing, but they are not using CTCs, the one item I am thankful for Pestell, because cancer was on the map with cydy before Pestell (they already had done animal studies), but CTCs I don't believe were. Getting BTD + safety would be a huge advantage, though BTD imo with FDA is always uncertain. I would have thought they would have given in combo and also mono, the weekly dosing alone is a breakthrough, the lower side-effects is a breakthrough, people dying of AIDS, leronlimab might save to me is a breakthrough, but FDA doesn't see it that way I guess, so who knows. Maraviroc has been used with more people being approved with HIV, so that might get through p2 faster than BMS. . Maraviroc will get the black box label though. Vicriviroc was discontinued with HIV partly due to safety issues. Here is their safety results on BMS-813160 posted with Type 2 Diabetes and Diabetic Kidney Disease. Early yet,, but couple SAEs, maybe or maybe not related, but if I remember correctly similar SAEs to Vicriviroc.
https://clinicaltrials.gov/ct2/show/results/NCT01752985
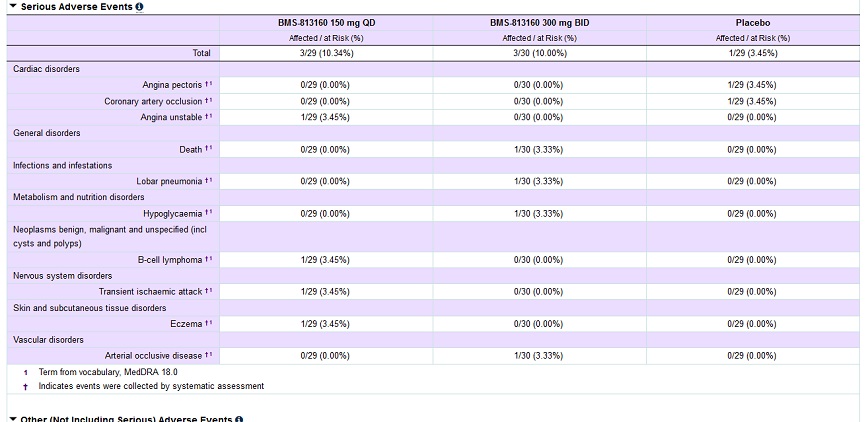
BMS trials,
https://clinicaltrials.gov/ct2/results?cond=&...bms+813160

 (0)
(0) (0)
(0)
 (0)
(0) (0)
(0)