GvHD Maraviroc p2 trial results. https://ww
Post# of 157023

https://www.sciencedirect.com/science/article...9118306013
Extended CCR5 Blockade for Graft-versus-Host Disease Prophylaxis Improves Outcomes of Reduced-Intensity Unrelated Donor Hematopoietic Cell Transplantation: A Phase II Clinical Trial.
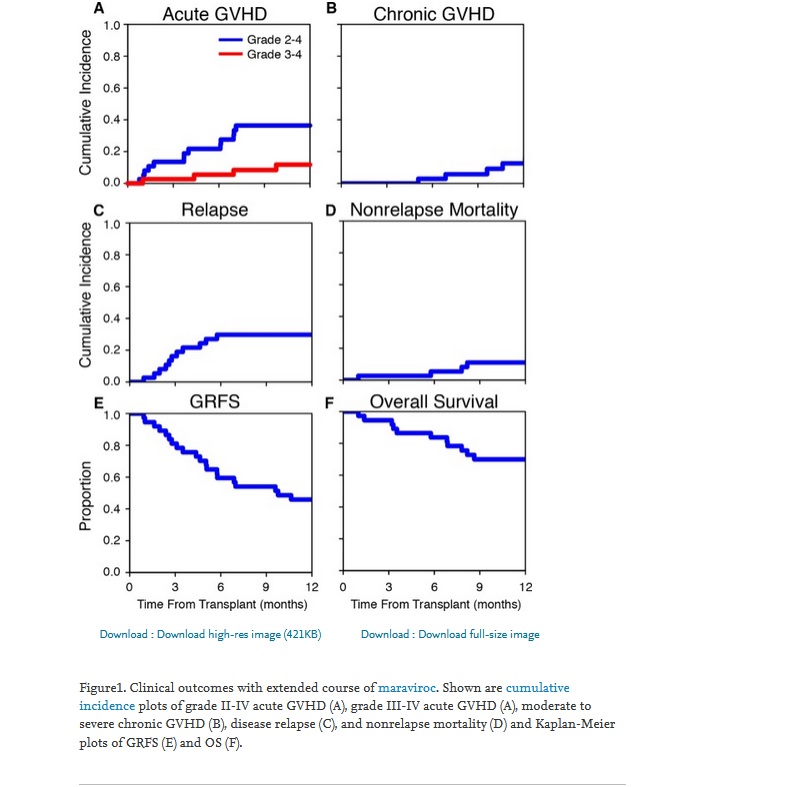
Quote:
The study met its primary endpoint, day +180 rate of grade II-IV acute GVHD, of 22 ± 7% (95% CI, 8% to 36%) (Figure1A), rejecting the null hypothesis of a rate of 52%. In addition, the day +180 rate of grade III-IV acute GVHD was 5 ± 4% (Figure1A). In the first 100 days, there were no cases of liver GVHD, 2 patients developed stage 1 upper GI GVHD, and 1 patient developed stage 3 lower GI GVHD together with aggressive relapse with documented leukemic infiltrates in the GI mucosa. At 1 year, the incidence of moderate to severe chronic GVHD was 8 ± 5%, NRM was 11 ± 5%, and disease relapse was 30 ± 8% (Figure1B to D). The GRFS rate at 1 year was 46 ± 8% and OS rate at 1 year was 70 ± 8% (Figure1E and F). With a median follow-up of 36.1 months, the median survival has not yet been reached. At the time of this report, 20 of 37 patients are alive and 19 are in complete remission.
also pediatric p2 study results
https://www.sciencedirect.com/science/article...9118310759
Pediatric Phase II Study of Maraviroc for Acute Graft Versus Host Disease Prophylaxis
Quote:
Results
Thirty-one patients were enrolled prospectively, 14 on the phase I and 17 on the phase II study.
Phase II study Demographics are shown in Figure 1. Median maraviroc trough concentrations were therapeutic at > 100 ng/mL on day+7 and day+14 (Figure 2A).
Functional CCR5 blockade was observed in all except one patient at day zero and day+14 (Figure 2B, 3A).
Two patients developed grade 1 acute skin GVHD and were successfully treated with corticosteroids. Four patients developed gastrointestinal (GI) GVHD (Upper GI GVHD n=2, lower GI GVHD n=2). Two of these 4 patients with GI GVHD did not receive the planned 34 days of maraviroc.
No adverse effects to maraviroc were observed. Seven patients discontinued maraviroc at a median of day+14 (range day+1- day+29) due to study rules regarding hepatotoxicity (n=5), renal function decline (n=1) and withdrawal from study (n=1).
Phase I± Phase II study Incidence of GI GVHD in all 31 patients who received maraviroc was 19% (n=6) compared to 22% in historic controls (p=0.29). Four of the 6 GI GVHD patients did not complete maraviroc treatment.
CD38 expression on CD4+ T-cells was elevated on day+30 in patients who developed GVHD compared to patients without GVHD (Figure 3B). We observed no difference in IL-15, CXCL10 and CXCL9 levels in patients with and without GVHD.
 (1)
(1) (0)
(0)