This week’s press release added to new words in
Post# of 158159

EMT and CAML
“no detectable circulating tumor cells (CTC) or EMTs in the peripheral blood”
“Other cancer-associated macrophage-like (CAML) cells in the blood sample”
EMT Epithelial–mesenchymal transition
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5228449/
Biomarkers for EMT and MET in breast cancer: An update
Metastasis and recurrence are the leading cause of mortality due to breast cancer, but the underlying mechanisms are still poorly understood. Understanding the breast cancer metastasis mechanism is important for early diagnosis and treatment of breast cancer. The seeding and growth of breast cancer cells at sites distinct from the primary tumor is a complex and multistage process. Recently, it has been reported that the epithelial-mesenchymal transition (EMT) and the mesenchymal-epithelial transition (MET) are the main mechanisms for breast cancer metastasis. During EMT, carcinoma cells shed their differentiated epithelial characteristics, including cell-cell adhesion, polarity and lack of motility, and acquire mesenchymal traits, including motility and invasiveness. This review has summarized the studies of known EMT biomarkers in the context of breast cancer progression. These biomarkers include EMT-related genes, proteins, microRNAs and kinases. In general, the findings of these studies suggest that EMT markers are associated with the invasion and metastasis of breast cancer. Further studies on the link between EMT markers and breast cancer will contribute to identify biomarkers for predicting early breast cancer metastasis as well as to provide new ideas for the treatment of breast cancer.
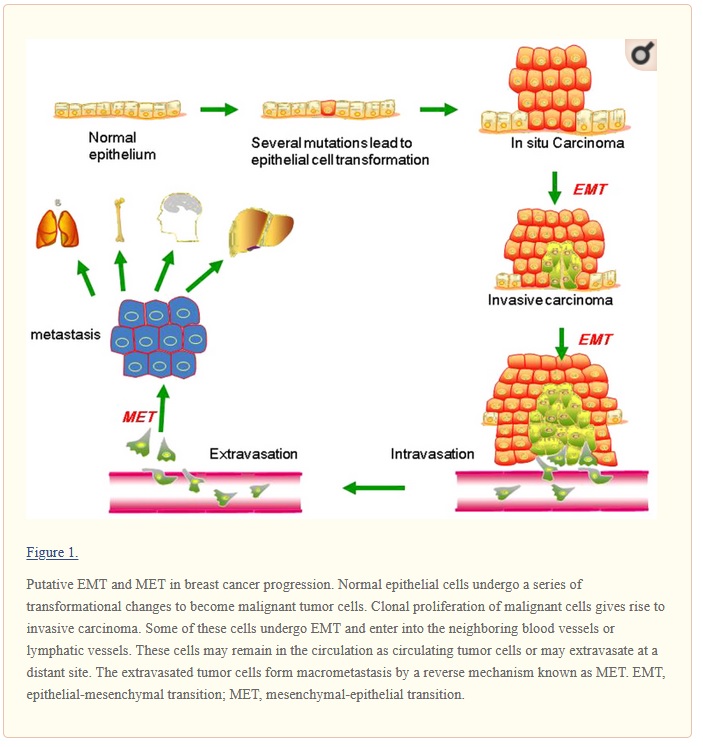
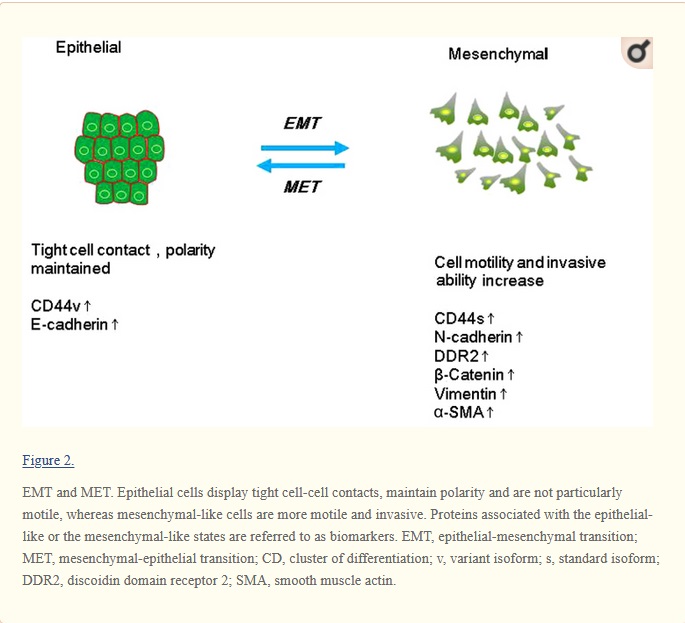
more discussion from wiki
https://en.wikipedia.org/wiki/Epithelial%E2%8...transition
In cancer progression and metastasis
Initiation of metastasis requires invasion, which is enabled by EMT.[37][38] Carcinoma cells in a primary tumor lose cell-cell adhesion mediated by E-cadherin repression and break through the basement membrane with increased invasive properties, and enter the bloodstream through intravasation. Later, when these circulating tumor cells (CTCs) exit the bloodstream to form micro-metastases, they undergo MET for clonal outgrowth at these metastatic sites. Thus, EMT and MET form the initiation and completion of the invasion-metastasis cascade.[39] At this new metastatic site, the tumor may undergo other processes to optimize growth. For example, EMT has been associated with PD-L1 expression, particularly in lung cancer. Increased levels of PD-L1 suppresses the immune system which allows the cancer to spread more easily. [40]
EMT confers resistance to oncogene-induced premature senescence. Twist1 and Twist2, as well as ZEB1 protects human cells and mouse embryonic fibroblasts from senescence. Similarly, TGF-β can promote tumor invasion and evasion of immune surveillance at advanced stages. When TGF-β acts on activated Ras-expressing mammary epithelial cells, EMT is favored and apoptosis is inhibited.[41] This effect can be reversed by inducers of epithelial differentiation, such as GATA-3.[42]
EMT has been shown to be induced by androgen deprivation therapy in metastatic prostate cancer.[13] Activation of EMT programs via inhibition of the androgen axis provides a mechanism by which tumor cells can adapt to promote disease recurrence and progression. Brachyury, Axl, MEK, and Aurora kinase A are molecular drivers of these programs, and inhibitors are currently in clinical trials to determine therapeutic applications.[13] Oncogenic PKC-iota can promote melanoma cell invasion by activating Vimentin during EMT. PKC-iota inhibition or knockdown resulted an increase E-cadherin and RhoA levels while decreasing total Vimentin, phophorylated Vimentin (S39) and Par6 in metastatic melanoma cells. These results suggested that PKC-ι is involved in signaling pathways which upregulate EMT in melanoma.[43][44]
EMT has been indicated to be involved in acquiring drug resistance. Gain of EMT markers was found to be associated with the resistance of ovarian carcinoma epithelial cell lines to paclitaxel. Similarly, SNAIL also confers resistance to paclitaxel, adriamycin and radiotherapy by inhibiting p53-mediated apoptosis.[45] Furthermore, inflammation, that has been associated with the progression of cancer and fibrosis, was recently shown to be related to cancer through inflammation-induced EMT.[46] Consequently, EMT enables cells to gain a migratory phenotype, as well as induce multiple immunosuppression, drug resistance, evasion of apoptosis mechanisms.
Some evidence suggests that cells that undergo EMT gain stem cell-like properties, thus giving rise to Cancer Stem Cells (CSCs). Upon transfection by activated Ras, a subpopulation of cells exhibiting the putative stem cell markers CD44high/CD24low increases with the concomitant induction of EMT.[47] Also, ZEB1 is capable of conferring stem cell-like properties, thus strengthening the relationship between EMT and stemness. Thus, EMT may present increased danger to cancer patients, as EMT not only enables the carcinoma cells to enter the bloodstream, but also endows them with properties of stemness which increases tumorigenic and proliferative potential.[48]
However, recent studies have further shifted the primary effects of EMT away from invasion and metastasis, toward resistance to chemotherapeutic agents. Research on breast cancer and pancreatic cancer both demonstrated no difference in cells' metastatic potential upon acquisition of EMT.[49][50] These are in agreement with another study showing that the EMT transcription factor TWIST actually requires intact adherens junctions in order to mediate local invasion in breast cancer.[51] The effects of EMT and its relationship to invasion and metastasis may therefore be highly context specific.
CAML
https://blogs.shu.edu/cancer/2015/04/29/cance...onitoring/
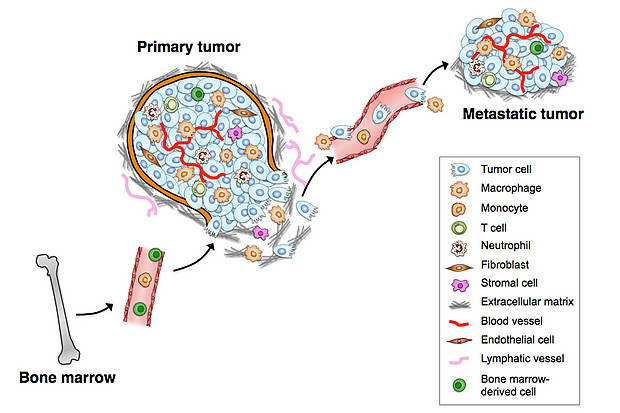
TAMs interact with tumor cells that undergo the EMT (epithelial-mesenchymal transition) and gain access to blood vessels, becoming circulating tumor cells (CTCs). Tumor-associated macrophages (TAMs) are specialized differentiated macrophages found within most tumors, which can be used as prognostic indicators of either tumor invasiveness or tumor suppression. TAMs, recruited to the stroma from circulating monocytes, are required for tumor cell intravasation, migration, extravasation, and angiogenesis. Tumors attract monocytes via chemoattractants (e.g., MCP-1, CCL-2). In turn TAMs secrete cytokines and growth factors (e.g., MMP-1, CXCL12) which stimulate tumor cells with the potential to become circulating tumor cells (CTCs). TAMs and CTCs then migrate via the lymphatic system or intravasate across intratumor capillary barriers into peripheral circulation.
But do TAMs and cancer cells continue to interact with each other outside of the primary tumor microenvironment?
YES.
It is believed that newly-discovered Cancer-Associated Macrophage-Like (CAML) cells are TAMs that accompany cancer cells into the circulation.
CAMLs were seen only in cancer patients –
CAMLs are involved in the phagocytosis of cancer cell debris –
CAMLs interact with CTCs (circulating tumor cells) –
Collectively, these data point to using CAML assays as “liquid biopsies” for the diagnosis, staging and monitoring of patients with cancer:
https://cancerres.aacrjournals.org/content/78...ement/2631
Cancer associated macrophage-like (CAMLs) cells in blood predict progression and survival for all stages of solid tumors
Background: We previously demonstrated that cancer associated macrophage-like cells (CAMLs) are cancer specific giant polyploid cells circulating in the blood of patients with solid tumors. Building on our initial discovery, others have shown that these hyperploidy cells are an innate immune response that is associated with decreased survival. However to date, no studies have been done to elucidate their clinical significance as in relation to the various stages of malignant disease. We established a 4 year prospective study of 315 patients from a variety of solid tumors (breast, prostate, lung, renal cell, pancreas, and esophageal) comparing the morphological properties of CAMLs in both early and late stage disease as they relate to progression free and overall survival (PFS/OS). These data suggest that CAML size appears have a strong negative correlation with progression and survival of cancer patients, irregardless of stage of disease, indicating their possible use as a non-invasive blood based biomarker in solid tumors.
Methods: A prospective multi-institutional study used anonymized peripheral blood from 315 cancer patients [stage I (n=62), stage II (n=72), stage III (n=69) & stage IV (n=106)] from subjects with breast (n=59), esophageal (n=27), lung (n=59), renal cell carcinoma (n=37), prostate (n=74), pancreas cancers (n=59). CAMLs were isolated by the CellSieve™ microfiltration technique at 5 institutions and stained for cytokeratin 8, 18, & 19, CD14 and CD45. After imaging, a size based threshold ≥50µm was used to separate the patient cohorts, based on previously published assessments.
Results: At least one CAML was found in 92% of cancer patients (n=289/315), with the lowest sensitivity in stage 1 (86%), followed by stage 2 (90%), stage 3 (97%) and stage 4 (95%). The property of CAML size was then evaluated. Overall, CAMLs of <50µm in size had superior PFS (HR: 3.8; 95% CI 2.8-5.3; p<0.001) and OS (HR=3.8; 95% CI 2.6-5.7;p<0.001) than those with ≥50 µm CAMLs. By each stage individually, stage 1 (PFS HR=7.5; 95% CI 3.2-17.7; p<0.001), stage II (PFS; HR=4.1; 95% CI 1.9-8.8; p<0.001), stage III (PFS HR=3.1; 95% CI 1.7-5.7; p=0.007), and stage IV (PFS HR=2.7; 95% CI 1.6-4.4; p<0.001).
Conclusions: In this first large scale prospective study of the clinical utility of CAMLs, it appears that they are present in every stages of invasive solid tumor malignancies, with prevalence increasing with stage. Interestingly decreased CAML size was found to be associated with improved outcome and longer PFS. These data suggest that CAML detection and size assessment constitute a new real time predictor of progression, and survival in both early and late stage disease indicating the need for additional validation studies.
 (3)
(3) (0)
(0)