UPDATED FEB 2013 ELTP Due Diligence Investor Info
Post# of 36681

UPDATED FEB 2013 ELTP Due Diligence Investor Info
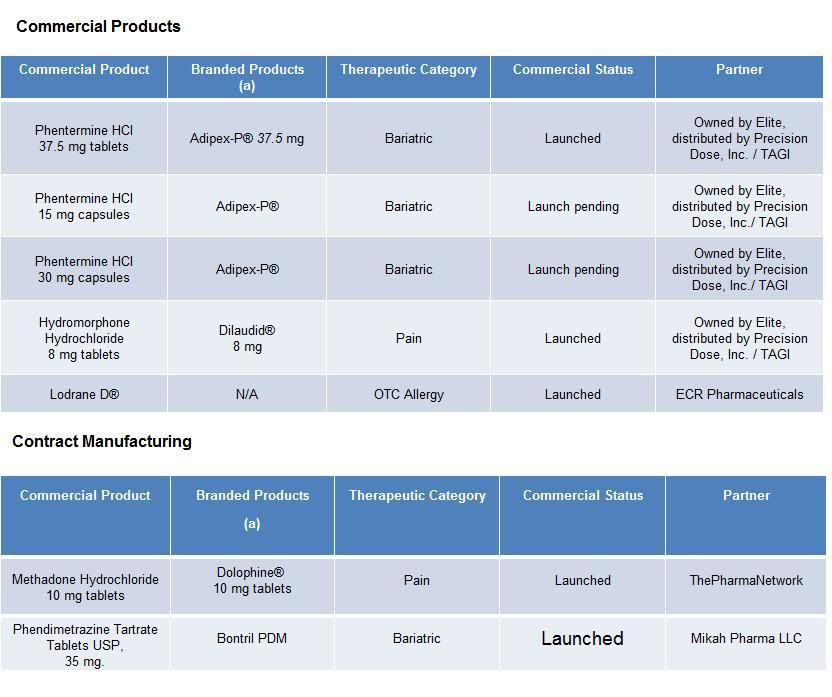
Elite Pharmaceuticals, Inc. develops oral sustained and controlled release products. Elite's strategy includes assisting partner companies in the life cycle management of products to improve off-patent drug products and developing generic versions of controlled release drug products with high barriers to entry. Elite has five commercial products with five additional products under review, pending approval by the FDA.
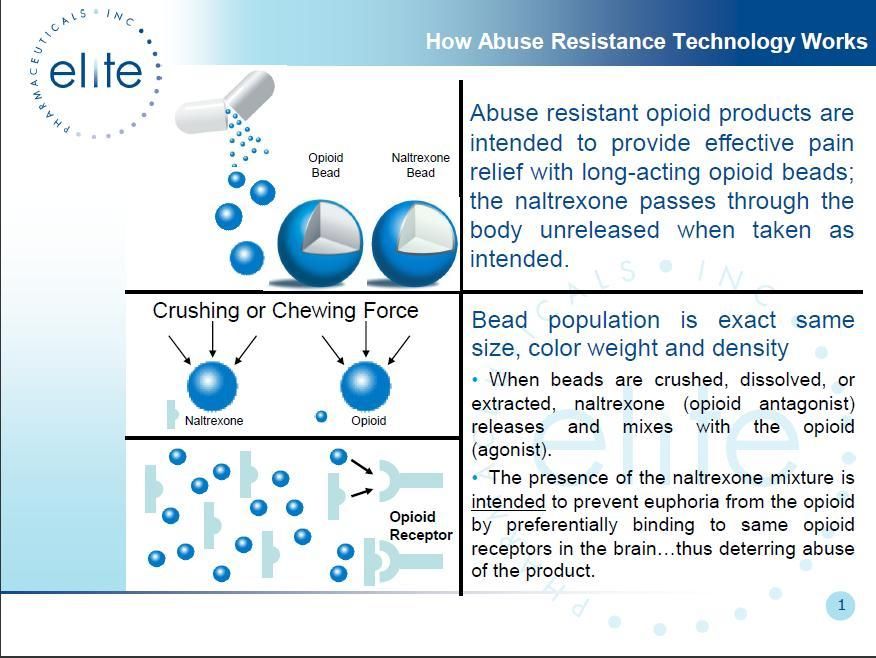
Elite’s lead pipeline products include abuse resistant opioids utilizing the Company’s patented proprietary technology, and a once-daily opioid. They are sustained release oral formulations of opioids for the treatment of chronic pain, which address two of the limitations of existing oral opioids: the provision of consistent relief of baseline pain levels and deterrence of potential abuse.
Elite also has partnered with Mikah Pharma to develop a new product, with Hi-Tech Pharmacal to develop an intermediate for a generic product, and a Hong Kong based company to develop a branded product for the United States market and its territories.
Elite operates a FDA GMP(Good Manufacturing Practices) and DEA registered facility for research, development, and manufacturing located in Northvale, NJ.
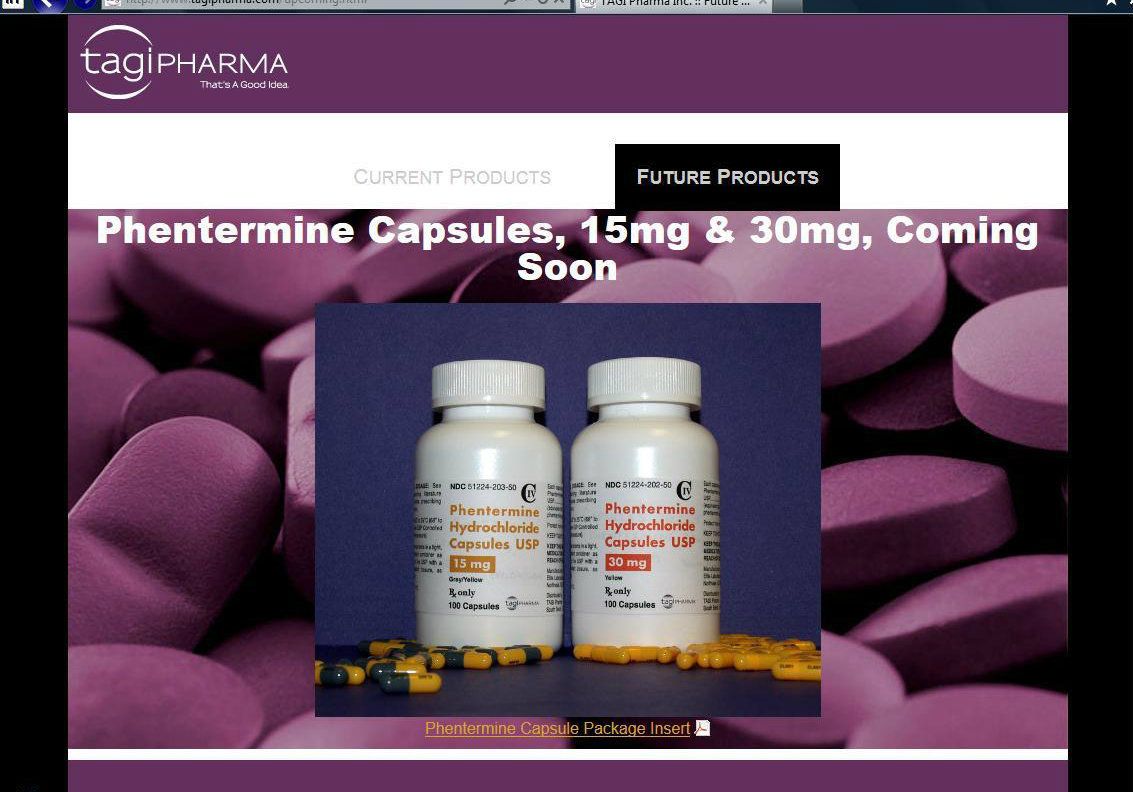

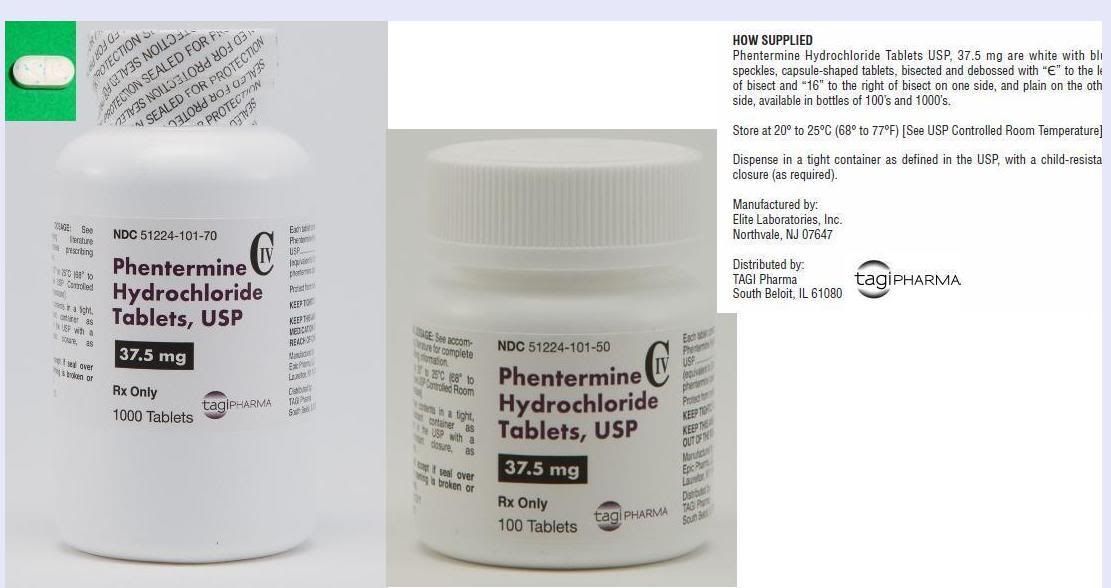
Link to 11 Billion dollar NYSE company - ACT- Actavis Labs distributing ELTP’s Phendimetrazine
http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=84198

Link to product pipeline:
http://www.elitepharma.com/product_pipeline.asp

Link to SEC filings:
http://investing.money.msn.com/investments/se...ymbol=ELTP
Recent PR's:
http://www.elitepharma.com/investor_relations.asp
12/13/2012 ELITE PHARMACEUTICALS, INC. ANNOUNCES AGREEMENT FOR SUPPLY OF PHENTERMINE ACTIVE INGREDIENT
11/14/2012 Elite Pharmaceuticals, Inc. Reports Financial Results for the Second Quarter of Fiscal 2013
...Revenues from new products continue to grow
PATENTS:
Since our incorporation, we have secured eight United States patents of which two have been assigned for a fee to Celgene Corporation for pulsed delivery of methylphenidate. Elite’s patents are:
PATENTS
U.S. patent 5,871,776
U.S. patent 5,902,632
U.S. patent 5,837,284 (assigned to Celgene Corporation)
U.S. patent 6,620,439
U.S. patent 6,635,284 (assigned to Celgene Corporation)
U.S. patent 6,926,909
U.S. patent 6,984,402
U.S. patent 8,182,836
We also have pending applications for four additional U.S. patents and four foreign patents. The pending patent applications are for an opioid agonist and antagonist products that Elite is developing to be used with controlled-release Oxycodone and other opioids to minimize the abuse potential for the opioids.
CEO Funding
Ceo Jerry Treppel personally invested 1 million dollars of his own money in 2 $500,000 non-dilutive financings to keep the research and products sales increases on target.
Elite Pharmaceuticals, Inc. ("Elite") (OTCBB:ELTP) announced today that it has amended the bridge loan agreement ("Loan Agreement") with Jerry Treppel, Elite's Chairman & CEO. Pursuant to the amendment to the Loan Agreement, Mr. Treppel has agreed to increase the maximum principal amount of the line of credit from $500,000 to $1,000,000. All other terms remain the same. The additional proceeds will be used primarily for working capital to support Elite's increase in production and growth of sales.
A copy of the amendment to the Loan Agreement will be filed in a Current Report on Form 8-K which will be filed with the Securities and Exchange Commission.
http://ih.advfn.com/p.php?pid=nmona&article=55384279
Outstanding shares :
As of November 8, 2012 the issuer had outstanding 350,337,260 shares of common stock
Transfer Agent:
American Stock Transfer & Trust Company, LLC.
6201 15th Avenue
Brooklyn, NY 11219
Phone: 800-937-5449
Fax: 718-236-2641
E Mail: info@amstock.com
Investor Relations Contact:
Dianne Will
(201) 367-7889
E Mail: dianne@elitepharma.com
http://www.elitepharma.com/ir_contact_us.asp
 (0)
(0) (0)
(0)