Why is FDA willing to approve this NASH drug with
Post# of 157747

Quote:
Why is FDA willing to approve this NASH drug with ORR of 11%, but is not willing to fast-track our BLA?
First Pro 140 does have fast track for BLA. The FDA is like the DMV, very very very slow on average. The average time from BLA submission to drug approval is 1.6 years. With fast track and rolling review, I’m expecting 3 to 6 months after BLA submission. Like the DMV, you can point and say hey they just got done, but reality is they probably waited years like you did also before walking out the door. ORR is an exception though, with possible approval on very little improvement. Why?
With results like this what’s not to love? N=50, 10mg, p value .8, nice right?
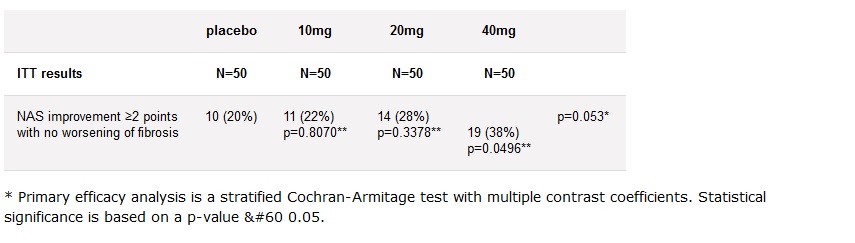
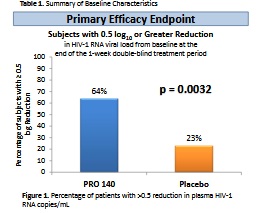
vs pro 140 combo at 350mg dose, p value .0032, FDA, “yeah let‘s ask for a higher dose”
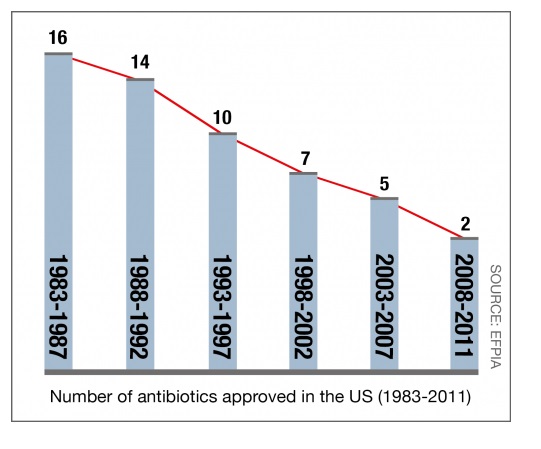
It is because there is no drug approved for NASH and several for HIV, even though people are still dying from AIDS; the FDA takes their eye off the ball. If this was 1980, Pro 140 would be approved yesterday, now they are not as concerned. Madness in my opinion.... Similar to antibiotics, no one wants to run the FDA gauntlet for smaller piece of the pie, which hasn‘t helped against the growing drug resistant bacteria problem.
Quote:
Each year in the U.S., at least 2.8 million people are infected with antibiotic-resistant bacteria, and more than 35,000 people die as a result.
Quote:
In 2016, 490 000 people developed multi-drug resistant TB globally, and drug resistance is starting to complicate the fight against HIV and malaria, as well.
Quote:
The Lancet Infectious Diseases contends that 40-50% of post-surgery bugs are already drug-resistant.
- The economics are upside-down. The less drugs people take, the better the drug works. Further, it takes on average, 23 years after the preclinical research stage for an antimicrobial to turn a profit. Thus, antimicrobial R&D is not cost-effective.
.
 (4)
(4) (0)
(0)