I believe nuGilead did have a connection. He came
Post# of 157013
The second one, chemokines have multiple pathways, you block one, and it activies through another.
From the latest Pestell paper, example rantes (CCL5) attaches to other receptor types. You block ccr5 and it might continue to activate through ccr1
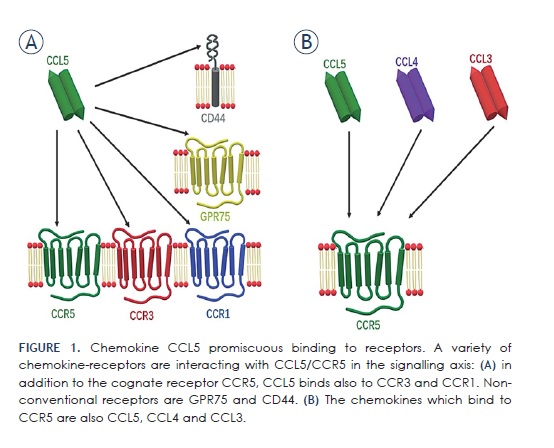
Quote:
This is not unusual, as many chemokine receptors display promiscuous ligand binding, meaning they have more than one highaffinity ligand.28 Such variety of CCL5 interactions causes the activation of multiple pathways and gives the ligand a diverse range of not only physiological, but also pathological functions, including in cancer.29
Quote:
In GB, Pan et al.29 reported that GB cell survivalalso involves CCL5 signalling, although interestingly not by binding to CCR5, but to an auxiliary receptor CD44, and in an autocrine manner triggers signalisation that inhibits apoptosis.
This is fact is what has happened when BP tried other receptor blocking, it worked in animal trials, but not human because of the multiple pathways.
Here is a dissertation on the subject from a previous post of mine,
https://investorshangout.com/post/view?id=5513400
Here is a dissertation that came out recently, 290 pages, good reading for anyone interested. I found it by chance, when I look at 2019 articles, I looked to see who is citing the article for the latest research.
“ Mapping the intracellular molecular mechanisms of chemokine signalling within cancer ”
https://ueaeprints.uea.ac.uk/id/eprint/71379/...d_Keil.pdf
Quote:
The importance of chemokine overexpression in cancer metastasis was first discovered in 2001fromthe seminal nature paper by Muller et al.(2001) [130].In thisstudychemokines CXCL12 and CCL19/21 werefound to be overexpressed at common sites of metastasis such asliver, lung, brain and lymph nodeswhilst their respective cognate receptorsCXCR4 and CCR7were overexpressed on various breast cancer cells [130]. Since thenCXCR4 overexpression has been further identified in at least 23 different cancer types and is often associated with a poor prognosis for cancer patients [261]. In addition to CXCL12other particularly well-studied chemokines within the cancer research field are CCL2 [262], CCL5 [263]and CCL19/21 [264]with emerging chemokine signalling axis of interest involving CXCL9/10/11-CXCR3 [265]and CXCL8-CXCR1/2 [266
Quote:
Chemokine Signalling: A Therapeutic Target for Cancer TreatmentGPCRs are the largest family of receptors in humans and their dysfunction underlies the pathogenesis of many diseases [280, 281]. To date, around 34% of all US Food and Drug Administration (FDA) approved drugs target GPCRs [282]. Successive preclinical in vitroand in vivocancer modelshave demonstrated the therapeutic benefit in targeting chemokine signalling in several cancers including breast [130, 283-286], prostate [287, 288]and leukaemia [289, 290]. Hencethere has been considerable interest from the pharmaceutical industry to develop and bring drugs which target the chemokine signalling network to clinical trial as a form of targeted treatment against cancer progression [291, 292].
Quote:
1.12.1 Small Molecules Targeting Chemokine Signalling in Cancer Patients CXCR4 is the most widely studied chemokine receptor in cancer, therefore it is unsurprising that most small molecules undergoing clinical trials are antagonists for CXCR4.
Challenges in Targeting Chemokine Signalling
Quote:
Mogamulizumab is the only licensed monoclonal antibody used to target a chemokine receptor in cancer treatment.
Quote:
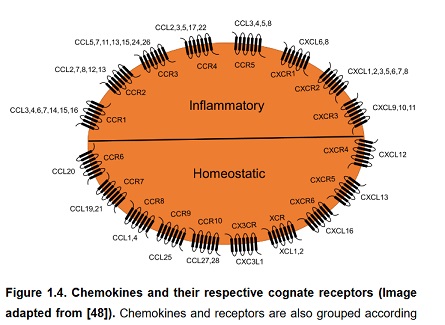
Despite nearly a decade of research into the therapeutic targeting of the chemokine signalling system for cancer treatmentonly two drugs worldwide have been licensed: AMD3100 and mogamulizumab,with only mogamulizumab used as a direct therapeutic agent against cancer progression. Several published review articles have discussed the challenges surrounding the blocking of the chemokine signalling system for the therapeutictreatmentof inflammatory diseases including cancer[291, 292, 317, 318]. One of the most commonly cited reason for clinical failure is the redundancy thoughtto underpinchemokine signalling [319, 320], which could be particularly problematic fortargeting chemokinesignalling axis associated with inflammation (figure 1.4) [48, 321].
 (2)
(2) (0)
(0)