Thanks BL. I feel RP has some explaining to do al
Post# of 157629

.
https://www.youtube.com/watch?v=T1kfh48pa3I&t=28m16s
RP:
28:16 we have FDA approval for this triple
28:19 negative breast cancer study and we
28:21 anticipate interim results within the
28:23 first half of this year
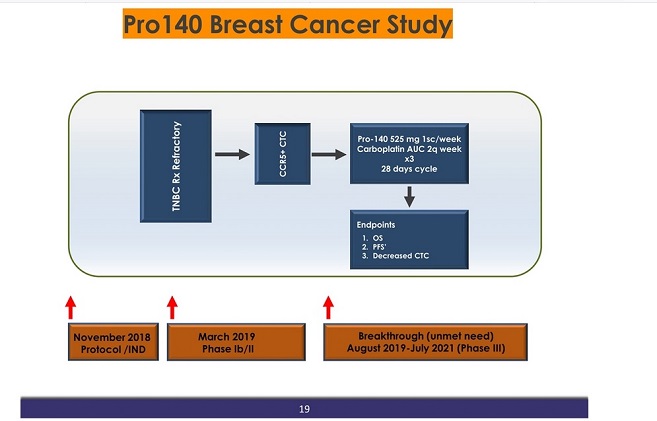
He also gave many talks and promoted this slide, I assume he created it, predicting phase 3 starting this month. Again contradicting 139. I hope the company has recorded communication from RP saying any day now; I suspect that is the case from misiu's previous posts.
Quote:
139 The FDA approved CytoDyn’s IND submission for the TNBC trial on November 23, 2018, and CytoDyn announced the initiation of the TNBC trial to the public via press release on November 26, 2018. However, the anticipated timelines provided in the press release – that the Company intended to “start the TNBC trial this year” (i.e., by the end of 2018) and dose patients during the first quarter of 2019 – were not accurate or reasonable, given that the typical time (per industry standards) needed for preparing, planning, and obtaining all necessary approvals for the trial in advance of being able to start accepting patients is closer to 11 months, not 1 or 2 months.
 (2)
(2) (0)
(0)