Personally, I don’t think so, being approved individual instead of linked together helps leonlimab become part of an optimized plan for R5 with combo. It is competition with leronlimab mono, it will get some market share, first to market, monthly dosing. The market is moving away from daily pills. But over 1.1M HIV in the US, with a goal to fix HIV, which they would need to get everyone on treatment. Mono leronlimab, being just one drug vs two or three, weekly self-dosing, low toxicity, would be very competitive in that environment if approved. I have more concerns about mono approval than I do mono competition, the FDA being a wildcard in something not done before. A BP that has fell behind the changing market, after looking at the data and what the FDA has requested with mono, if they felt it looked promising, might jump on the leronlimab train to catch-up very quickly, because monotherapy would be big news in the HIV world, tied to weekly self dosing, low toxicity, a big splash, and it would grab some market share, in my opinion. It is a big market, 3% would get 1B annual revenue in US alone, it will higher a higher penetration then that. All in my opinion of course.
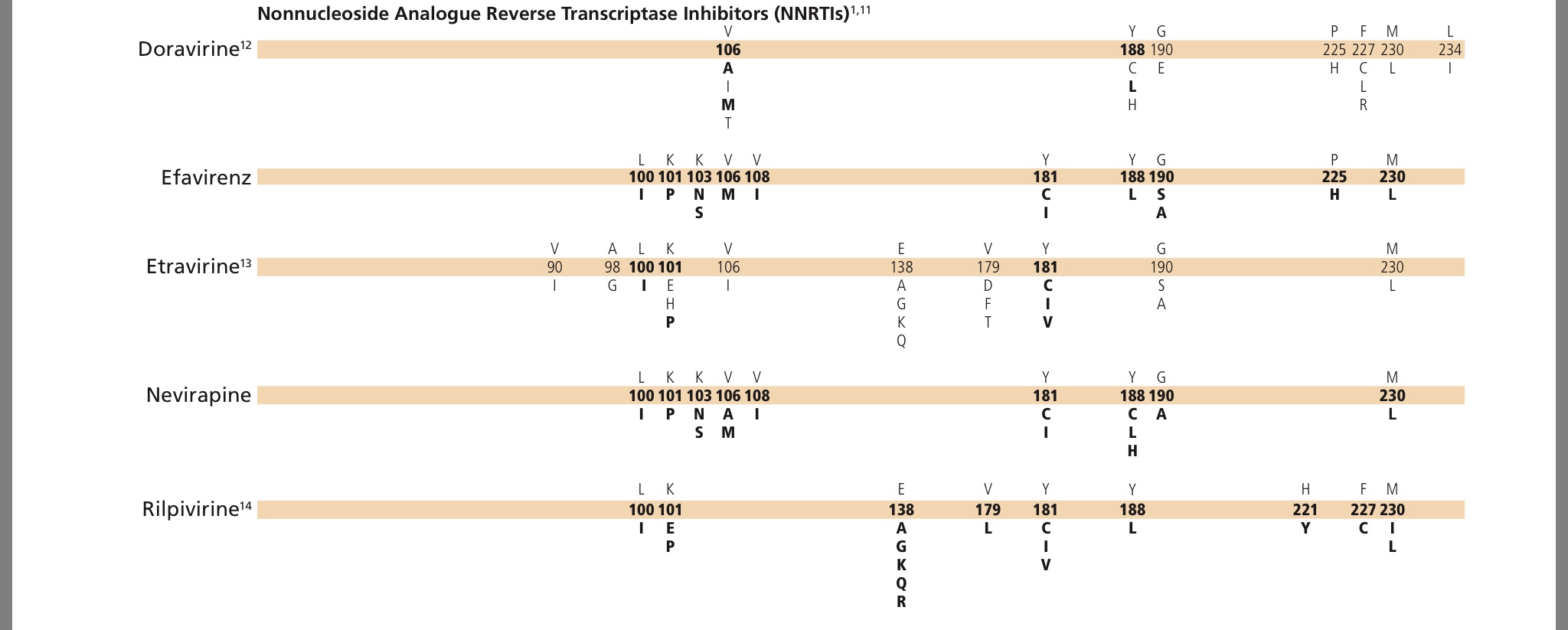
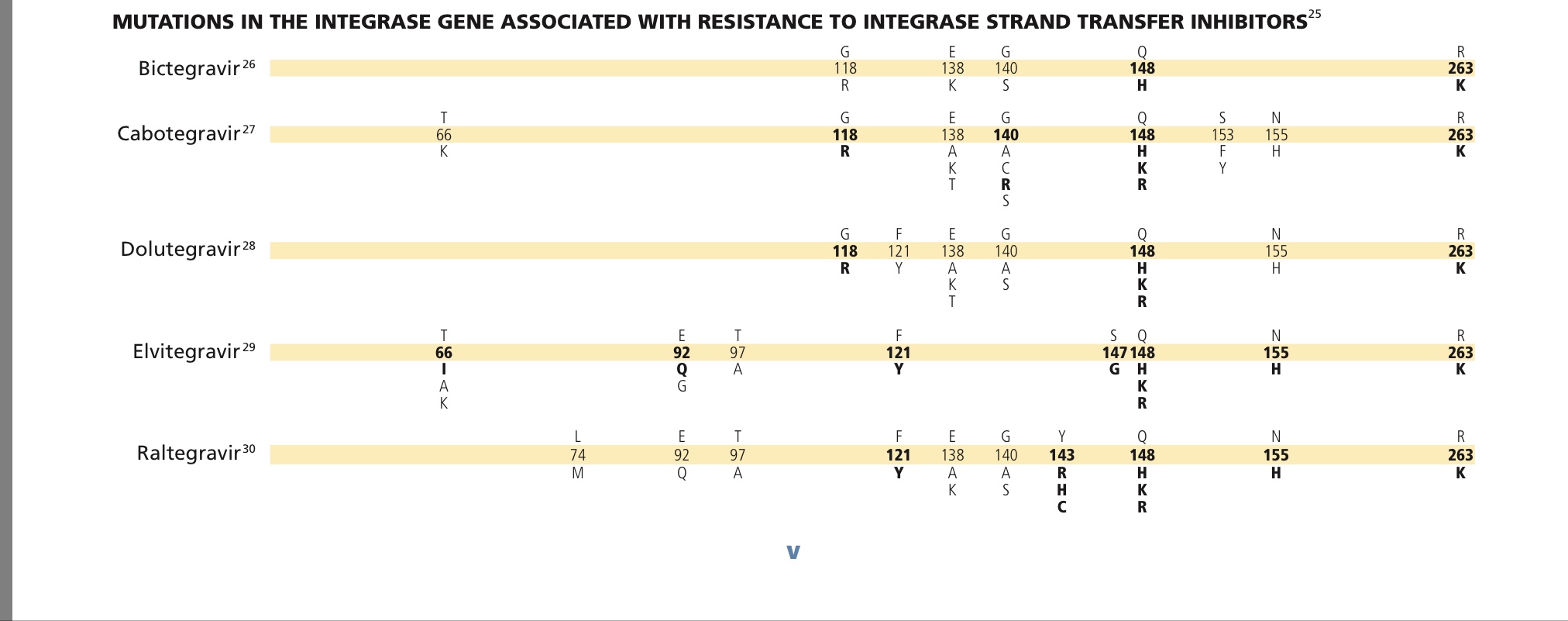
https://www.iasusa.org/wp-content/uploads/201...igures.pdf



 (1)
(1) (0)
(0)


 (1)
(1) (0)
(0)