Out most of today with my son, whose birthday is t
Post# of 157664

Here is the results of the survey, 31 people took the survey.
Question 1: Two was most selected, but given that almost half selected 3 or 4 (and no one selected 1), I feel 3 is the answer that matches this data the best.
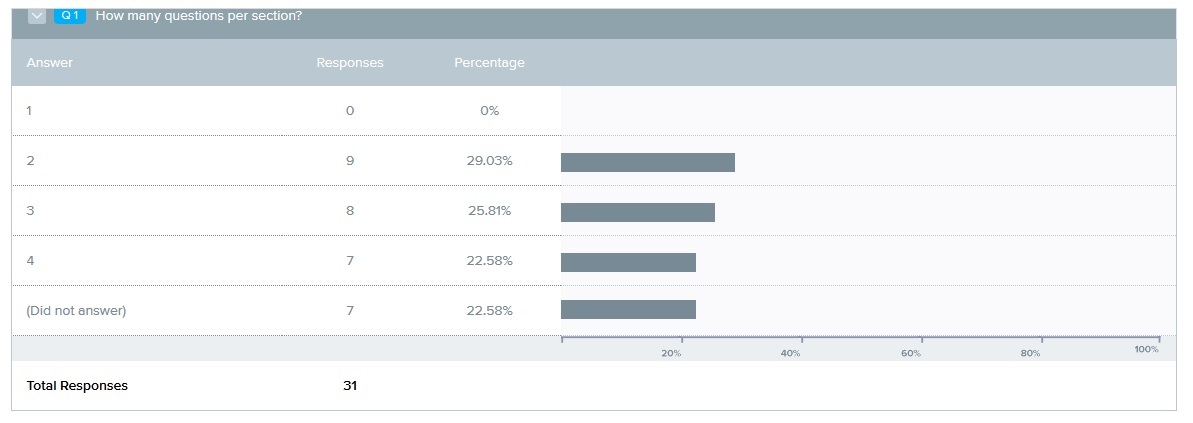
Question 2: The BLA timeline was the top choice. The top three questions by a wide margin all were related to safety data: when will the 700mg data be done, anticipated date the FDA will decide on 525 vs 700, and the timeline based on their decision.
Question 3: For Mono, when the FDA meeting is expected to occur was ranked #1 question by 74% of respondents. It won by such a wide margin, I wonder if that should be the only question asked for emphasis.

Question 4: For TNBC, the top question asked for an updated on the open site. The top 3 ranked questions by a wide margin all focused on the status of clinical site: why the delay in enrollment at the open site, why the delay in the remaining sites from opening.

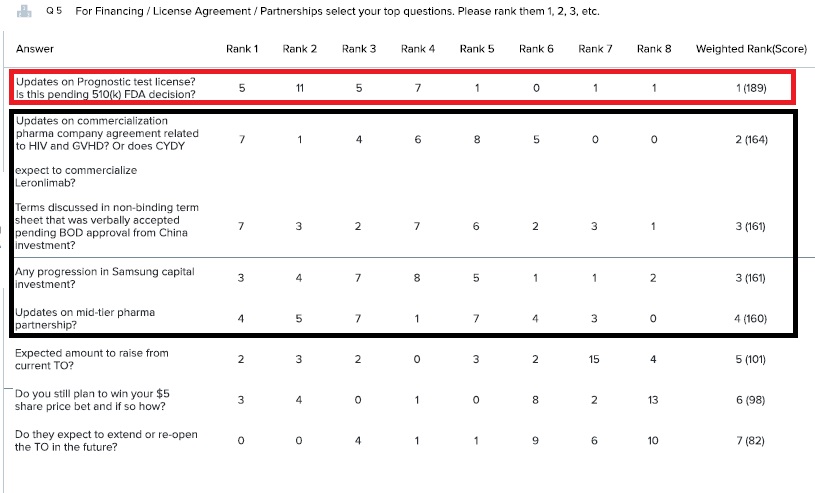
Question 5: The top ranked question by far was the Prostate Test Licensing. But the next 4 ranked questions, which almost tied, where all ready to licensing or partnerships. People were not as interested in TO information. Maybe since these top 5 ranked questions are all related, we can condense them down to three question and include them all somehow.
Question 6: Top ranked questions: impact from Dr. Rae, plan to increase share price, when will Dr. Sacha's research be published (which I believe NP has already said end of year). People were not as interested overall with how they plan to uplist at this time.

I didn't put the one status question with GvHD, since it was just the status of a lot of items rolled up into one question. With that, the questions listed in ranked order, which my need some wordsmithing before we are done.
Combo/BLA
BLA submission timeline with 525mg vs 700mg?
Anticipation date FDA will give final safety data requirements?
When will the 700mg safety data be complete?
Mono
Update on FDA meeting date?
TNBC
Recruiting status on the open TNBC site? Patients screened yet?
Any update on your investigation into the TNBC trial delays?
Updates on remaining sites opening? IRB approval for these yet?
Remaining (GvHD, Cancer, NASH, MS )
Status updates: GvHD enrollment, NASH study, any of the 8 cancer pre-clinical trials started, Colon Cancer IND, MS plans.
Financing / License Agreement / Partnerships:
Updates on Prognostic test license? Is this pending 510(k) FDA decision?
Updates on commercialization pharma company agreement related to HIV and GVHD? Or does CYDY expect to commercialize Leronlimab?
Terms discussed in non-binding term sheet that was verbally accepted pending BOD approval from China investment?
Any progression in Samsung capital investment?
Updates on mid-tier pharma partnership?
Other:
Impacts of Dr. Rae (Business Development) so far?
How does he plan to increase share price?
Expected release date for Dr. Sacha HIV/Leronlimab research results?
 (0)
(0) (0)
(0)