Just how common is missed deadlines in biotech? I
Post# of 157808

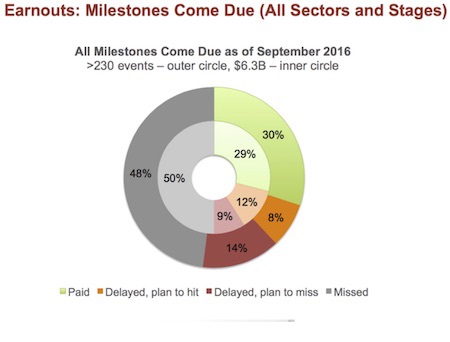
Here is a case study that might relate to the mTNBC trial; we might be dealing with similar issues here.
https://medsource.com/wp-content/uploads/2018..._Study.pdf
Quote:
Since 22 study sites had already been selected and qualified for the original study, MedSource knew that using a subset of those sites on the sister study would eliminate the time required to find and qualify new sites with an adequate patient population to support the study. However, the sites participating in the original protocol were large academic institutions with lengthy contracting processes, multiple scientific review committees, and local institutional review boards (IRBs). Maneuvering through study startup at these sites could typically take up to 18 months, but MedSource had planned and developed strategies that would streamline efforts across multiple channels. By reviewing the previously submitted feasibility surveys, MedSource was able to identify a subset of potential sites to participate in an abbreviated feasibility for the new study.
 (2)
(2) (0)
(0)