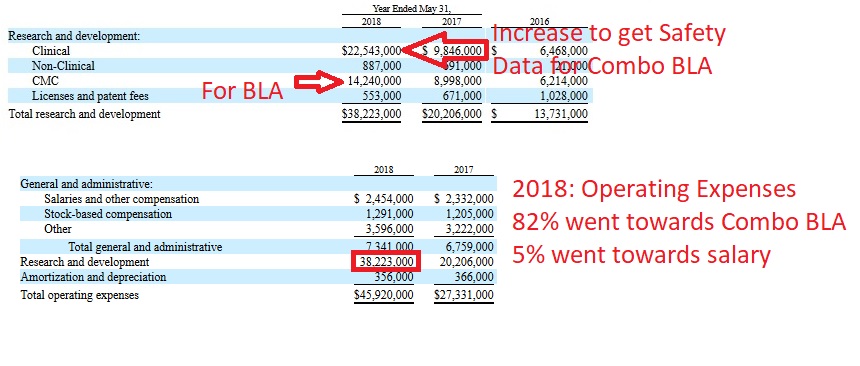
The majority of the funds raised has went towards getting Combo approved, as this is their number 1 priority. The current animal studies in NASH, Cancer, the TNBC trial and GvHD trial are very small; at most a couple million spent on these so far. Outside GvHD and TNBC, the others right now are being discussed to add value to the overall product (I'm assuming in BO or licensing talks). The biggest priority and largest cost has always been combo with the safety data the biggest cost. Nothing can be done right now to speed up Combo, we wait on the safety data, the trial is done. So as we wait, we discuss these other items, paying a small amount of money to fund NASH and the cancer mice data, which could add great value for the small amount spent. But, make no mistake, Combo is the biggest priority, because it is the path to approval. It is not being discussed because until we get the FDA's answer on the safety data (525mg or 700mg), there is nothing to discuss. Behind the scenes though lots of work is being done for the BLA.
Mono p3 investigatory started Dec 2016, but enrollment didn't ramp up until the FDA required 300 patients at 350mg in late 2017 for the combo safety data (look at the spike in clinical cost after--from 2017 to 2018)
https://www.cytodyn.com/media/press-releases/...ients-with
https://www.cytodyn.com/media/press-releases/...on-therapy
Quote:
Oct 2017 (FDA required 300 safety data, most coming from mono )
The FDA also confirmed that 300 patients will be required for the safety analysis in a BLA, which can be provided by all of the Company’s HIV trials, providing that those patients have been on a PRO 140 therapy for 24 weeks.


 (2)
(2) (0)
(0)