The clinical portion I believe is ready to go if t
Post# of 157910
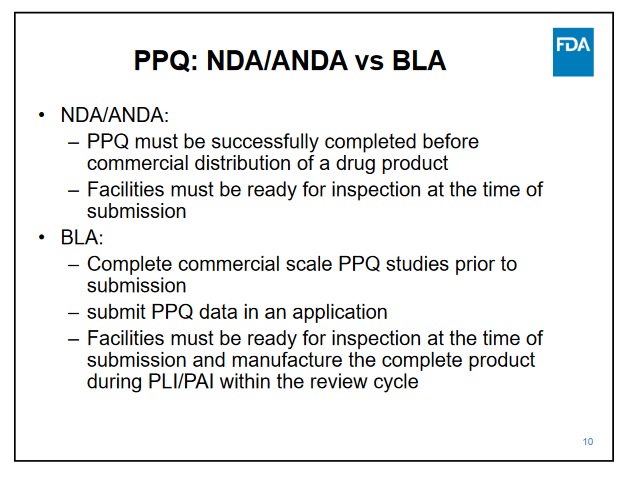
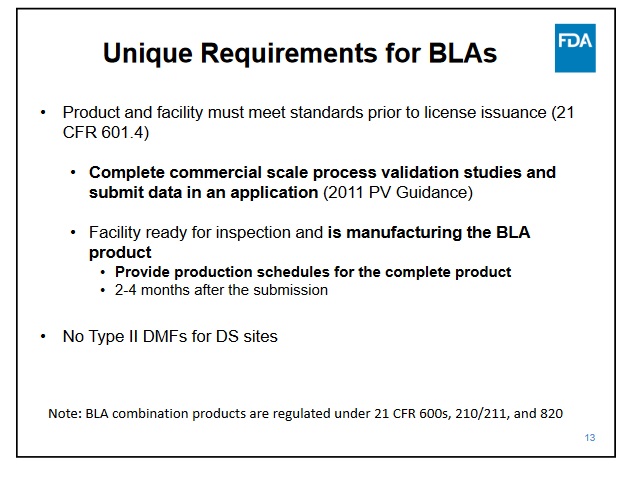
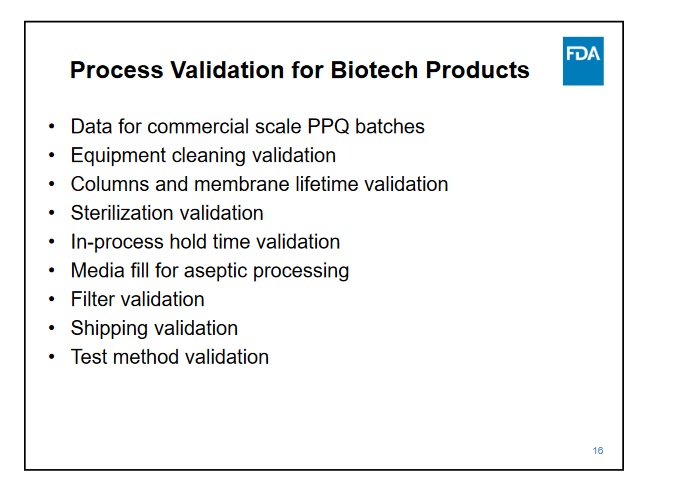
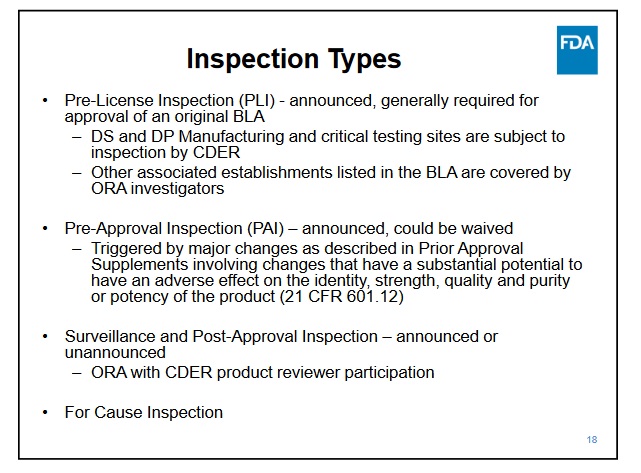
For the CMO portion, I'm not sure what all needs to be done, but NP said that would take a couple months. I guess they need to add Samsung data, so it might take some time to get the studies due at the time of BLA submission; it must be already being created and ready for inspection when the BLA is submitted.
Samsung has top notch facilities was approved in the last couple of years by the FDA for mabs and they have had several approvals this year for biosimilars. So that is just a matter of doing the work needed for the BLA and inspections.
2017
https://www.biopharma-reporter.com/Article/20...000L-plant
The world’s largest single bioproduction plant has received its first US regulatory approval to make a monoclonal antibody, says CDMO Samsung Biologics.
2018
https://www.prnewswire.com/news-releases/sams...54138.html
Samsung BioLogics announced license-approval by the US FDA to create a mAb drug product at its Plant 1 facility.
https://www.fiercepharma.com/manufacturing/sa...rean-plant
Samsung BioLogics reaches milestone with FDA nod for production of mAb at South Korean plant
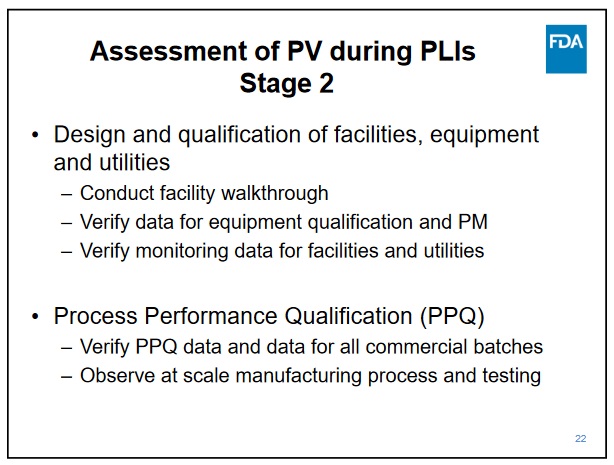
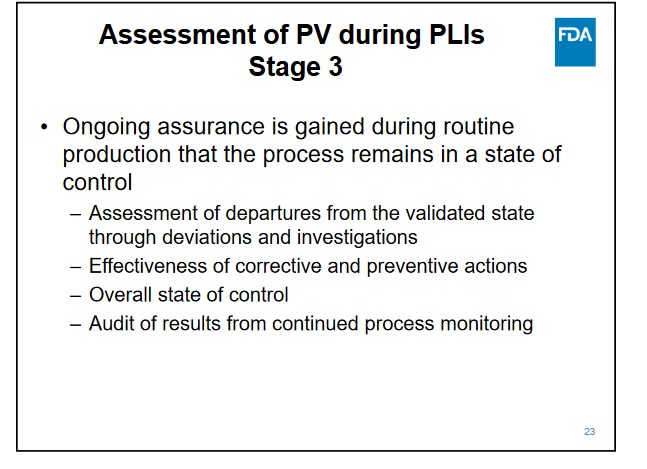
https://www2.ispe.org/imis/conference-handout...oducts.pdf
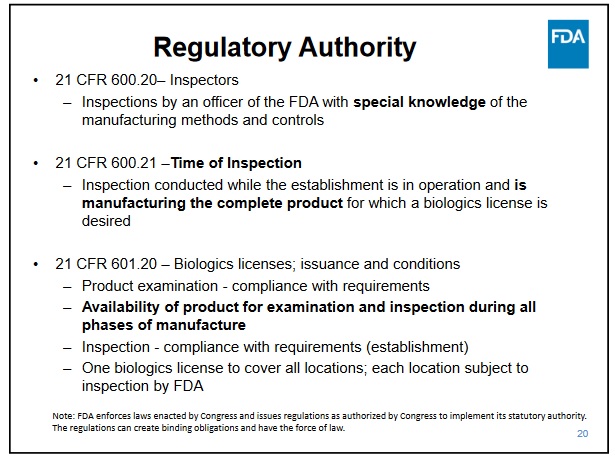
https://www.fda.gov/media/71021/download

 (2)
(2) (0)
(0)