Okay here is what we did have, I've marked out the
Post# of 157833

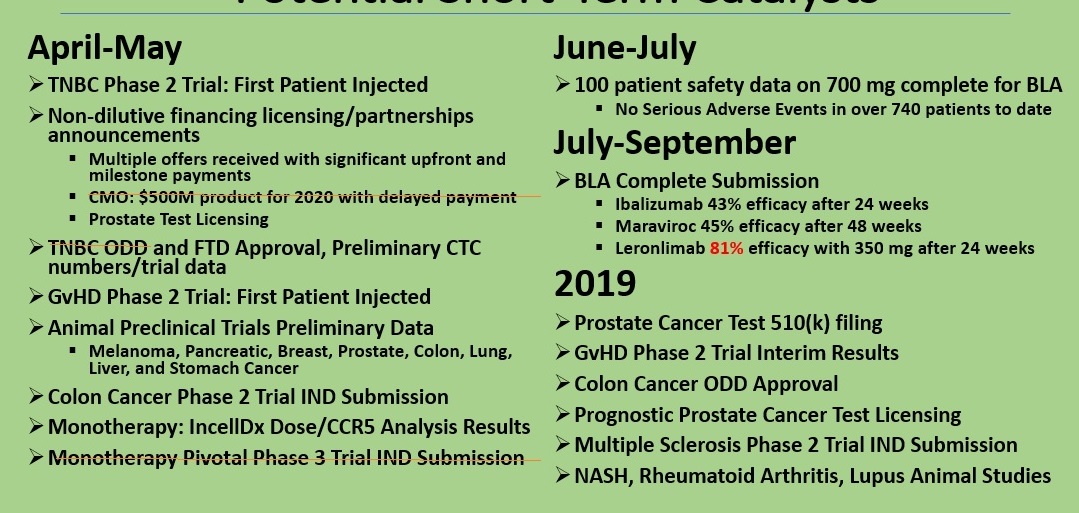
CMO: $500M product for 2020 with delayed payment
TNBC FTD Approval
Monotherapy Pivotal Phase 3 Trial IND Submission
Remaining (just copied over)
TNBC Phase 2 Trial: First Patient Injected
Non-dilutive financing licensing/partnerships announcements
Multiple offers received with significant upfront and milestone payments
Prostate Test Licensing
TNBC ODD Approval
TNBC Preliminary CTC numbers/trial data
GvHD Phase 2 Trial: First Patient Injected
Animal Preclinical Trials Preliminary Data: Melanoma, Pancreatic, Breast, Prostate, Colon, Lung, Liver, and Stomach Cancer
Colon Cancer Phase 2 Trial IND Submission
Monotherapy: IncellDx Dose/CCR5 Analysis Results
Monotherapy Pivotal Phase 3 Trial IND Submission
100 patient safety data on 700 mg complete for BLA
No Serious Adverse Events in over 740 patients to date
July-September
BLA Complete Submission
2019
Prostate Cancer Test 510(k) filing
GvHD Phase 2 Trial Interim Results
Colon Cancer ODD Approval
Prognostic Prostate Cancer Test Licensing
Multiple Sclerosis Phase 2 Trial IND Submission
NASH, Rheumatoid Arthritis, Lupus Animal Studies
Milestones times for last presentation.
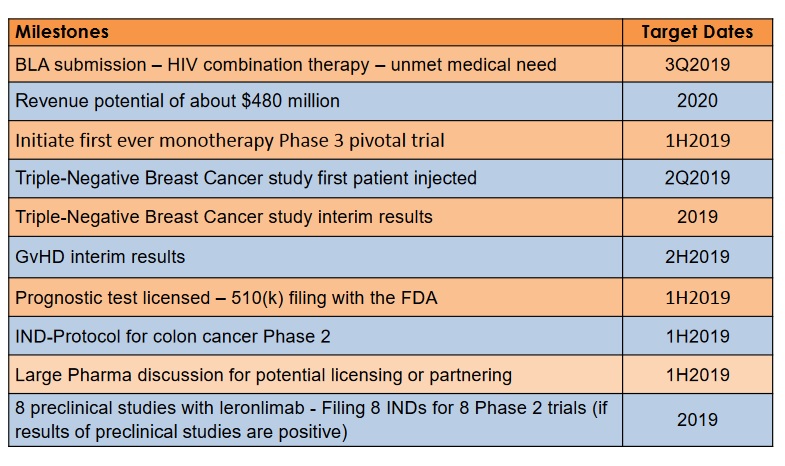
My added ones, any more?
Mono pivotal trial approval
Cash fund for 1 year (no more dilutive financing)
Stopping Conversions on Note (by paying it off or having cash for monthly payments)
FDA response to use 525mg safety data with Combo
Results from NASH mice study.
 (2)
(2) (0)
(0)