I agree and wished they would have publicly addres
Post# of 158052
Over the past couple of days I had some time while driving, so I listened to a couple of their recent presentations again. One theory for the delay I have is that with the CTC levels / tumor reduction (secondary/surrogate endpoints) they expect Accelerated Approval (based on discussions with FDA of course) and any delay in combo approval impacts cancer also. I am not sure if this theory holds water, but until proven otherwise, I believe that cancer results are going to be great and the delay is somehow justified. I was reading that drugs can be approved based on surrogate endpoints (especially cancer) such as reduced CTC levels / tumor shrinkage while results are provided to the FDA post-approval during a required confirmatory trial period.
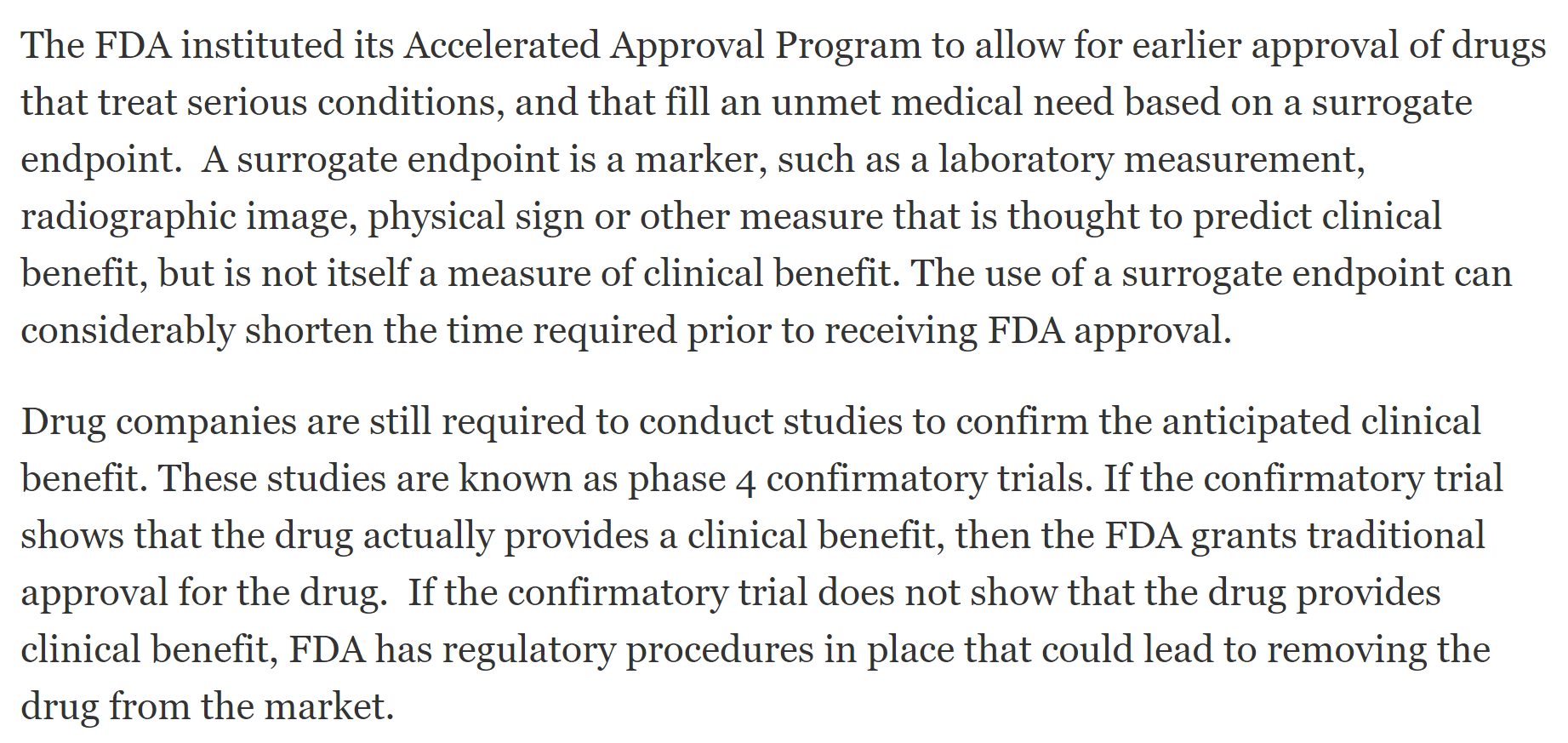
https://www.fda.gov/drugs/information-healthc...al-program
Here is a few excerpts from the FDA "Expedited Programs for Serious Conditions" guide that further explain Accelerated Approvals:
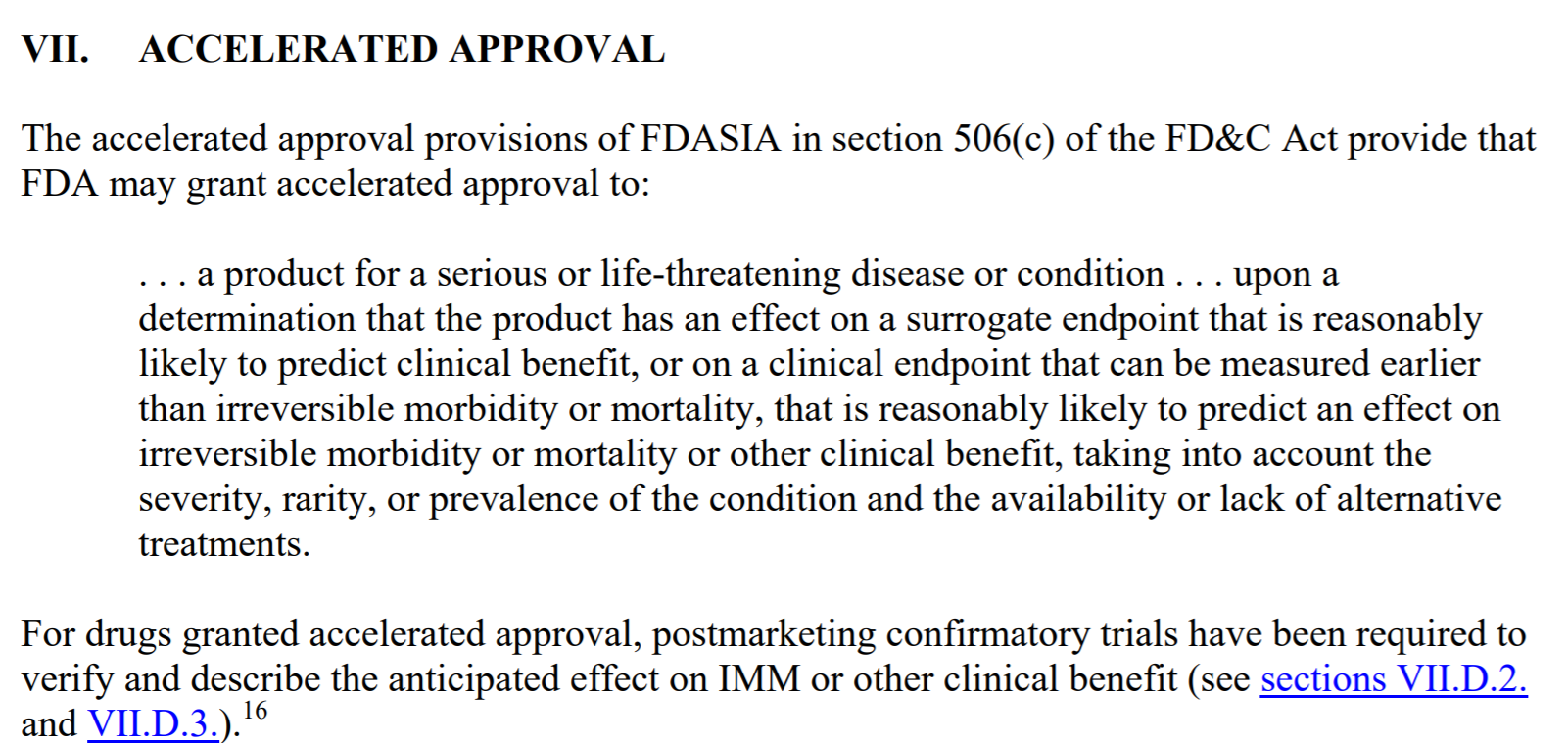
I also thought this quote was fitting to our situation: "FDA encourages sponsors to communicate with the Agency early in development concerning the potential eligibility of a drug for accelerated approval, proposed surrogate endpoints or intermediate clinical endpoints, clinical trial designs, and planning and conduct of confirmatory trials. A sponsor seeking accelerated approval may also need to prepare for a more rapid pace for other aspects of the drug development (e.g., manufacturing (COMPLETE), development of a necessary companion diagnostic (CTC BLOOD TEST).
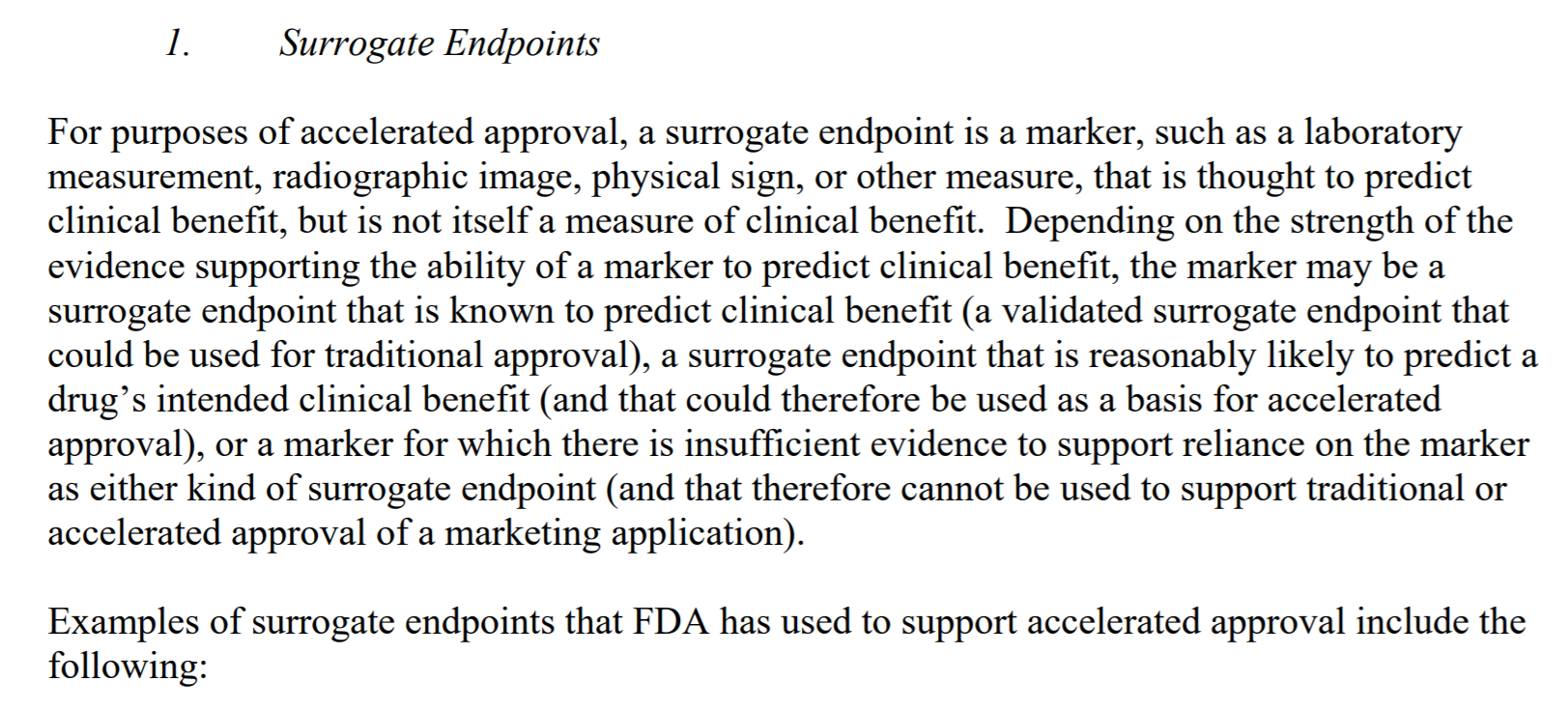 ......Radiographic evidence of tumor shrinkage (response rate) in certain cancer types has been considered reasonably likely to predict an improvement in overall survival.
......Radiographic evidence of tumor shrinkage (response rate) in certain cancer types has been considered reasonably likely to predict an improvement in overall survival. and:Surrogate endpoints are often thought to be a measure of the following, for example: ......An effect that predicts the ultimate outcome (e.g., tumor shrinkage could be expected to delay symptomatic progression and improve survival
https://www.fda.gov/media/86377/download
Maybe they spent this time to fine tune the trial or the FDA needed further clarification, validation or proof of concept on the CTC test?
How fast are such Accelerated Approvals? I'm not sure, but if they meet the criteria it may be fast as these approvals are based on serious diseases and unmet needs and the FDA can pull approval later if confirmatory trials prove different results.
Regardless, the silence is frustrating and likely plays a part of the share price decline recently. I hope we get some clarification tomorrow on many fronts.[/i]
 (0)
(0) (0)
(0)