I just looked at the clinical trial site and agree
Post# of 157779
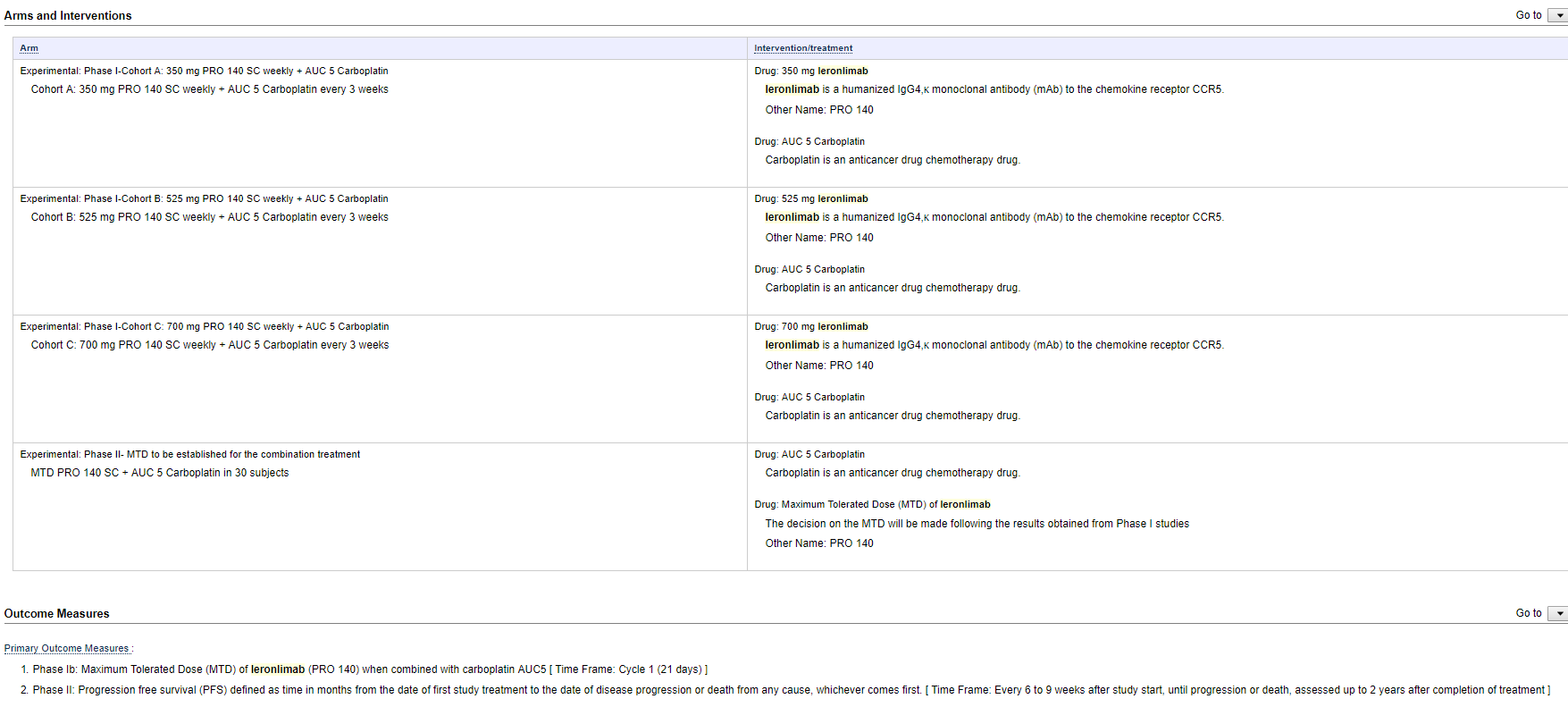
It states each cohort (A at 350 mg, B at 525 mg and C at 700 mg) is the respective dosage of PRO 140 weekly and AUC 5 Carboplatin every 3 weeks.
The Primary Outcome Measures for Phase 1b: "Maximum Tolerated Dose (MTD) of leronlimab (PRO 140) when combined with carboplatin AUC5 [ Time Frame: Cycle 1 (21 days) ]."
I understand this to mean the entire phase will only be 1 cycle and last 21 days. I guess it could possibly be interpreted as each cycle is 21 days with multiple cycles....but that doesn't align with how they describe the Phase II "Time Frame: Every 6 to 9 weeks after study start, until progression or death, assessed up to 2 years after completion of treatment."
 (0)
(0) (0)
(0)