Yes, here is the feb 1st PR again and slide 6 in t
Post# of 158016
So they added >30 on mono 700mg in February.
They had Feb 1st for mono:
45 (started 700mg) + 29 (switched to 700mg)
They added at least 30 in February, probably all 40 in screening but we know they have > 45+29+30=104 on mono 700mg, not counting the 30 in the combo extended trial they switched over to 700mg.
The final date will depend on when the enrolled these 30, if the fda will allow combo (which I assume they switched on feb 1st), or if they will allow less than 24 weeks of data. 24 weeks from now is the middle of August, since this pr was over a month old, we are looking at around July 15-August 15 for this safety data to be done if they FDA requires the full length and the full 100.
Any doubt now they have not been pushing full steam ahead when it comes to finishing combo?
Quote:
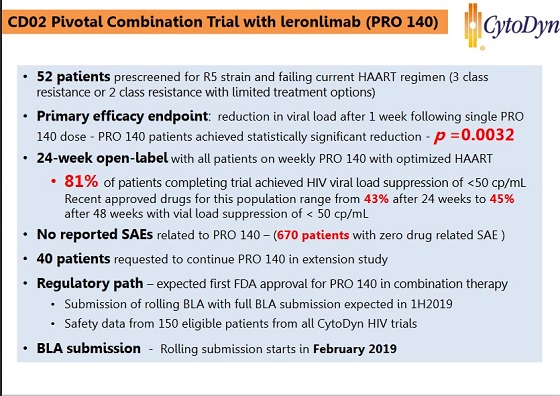
the FDA agreed to accept safety data from 100 patients in the monotherapy trial with the 700 mg dose, which enables the BLA submission for the combination therapy to use 700 mg instead of the original 350 mg dose.
CytoDyn currently has 45 patients enrolled in monotherapy with 700 mg, 29 additional patients who have switched from lower doses to the 700 mg dose and over 40 patients currently in screening to initiate 700 mg dose arm of monotherapy trial. The FDA also gave CytoDyn permission to upgrade all ongoing patients who are currently on 350 mg in the combination therapy to 700 mg dose.
 (1)
(1) (0)
(0)