A couple other notes on combo from the recent pres
Post# of 158786
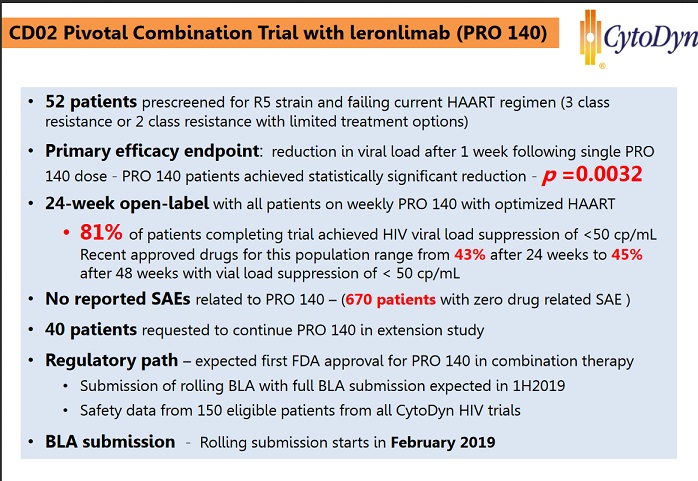
It met endpoint with 81% suppression, 40/49 people in study.
40 moved to extension, but NP said at 7:30 about 30 still in extension, so that is ~60% possibly now
He said at 8:00 the have more news to PR from FDA meeting about BLA on combo, but wanted to get the fda minutes to list correctly.
He said at 33:50 that they said March, but based on the FDA meeting, the will have to PR a ’change’ in timelines. I listened several times, I believe he said change, could have said ’raise‘ or ’revise’, not 100% sure. It might not be bad news, it could be moved up since the slide said rolling BLA starts in February.
http://noble.mediasite.com/mediasite/Play/a08...b6137a611d
slides: https://content.equisolve.net/cytodyn/media/9...fea2ae.pdf
 (0)
(0) (0)
(0)