IPIX Clinical Trial Summaries B-OM (Phase 2)
Post# of 72451

B-OM (Phase 2)
Kaplan-Meier Curves
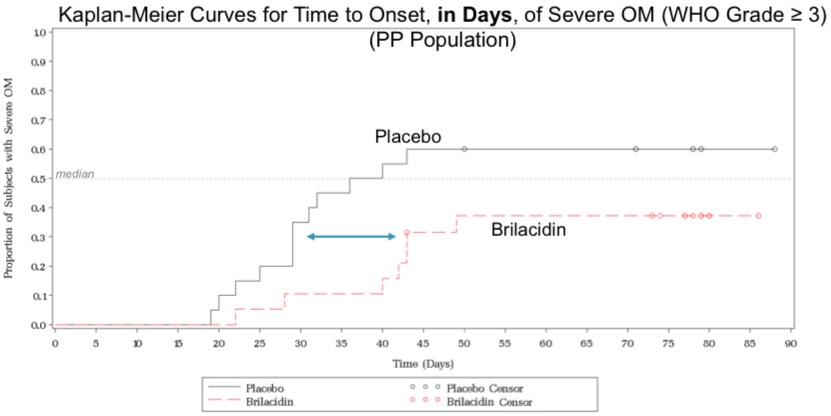
"Onset of Severe Oral Mucositis: Based on Kaplan-Meier curves, Brilacidin-OM oral rinse showed a clear separation from placebo in delaying the onset of SOM—particularly the period from approximately 28-42 days, after the initiation of treatment, during which the incidence of SOM rose strikingly in the placebo group while not in the group being treated with Brilacidin."
http://www.ipharminc.com/press-release/2018/1...ositis-som
Topline Results
· Brilacidin met primary endpoint of reduced incidence of severe OM experienced by patients during radiation therapy.
· Incidence of severe OM in Modified Intent to Treat (mITT) Population: Brilacidin 42.9%, Placebo 60.0%.
· Incidence of severe OM in Per Protocol (PP) Population: Brilacidin 36.8%, Placebo 60.0%.
· Trial results support continued and expedited development of Brilacidin-OM.
http://www.ipharminc.com/press-release/2017/1...r-patients
Subgroup Analysis
“For the Modified Intent-to-Treat (mITT) population, Brilacidin-OM in the aggressive chemotherapy regimen reduced the incidence of SOM by 65.0% ([incidence control- incidence active]/incidence control) as compared with placebo (Brilacidin: 25.0%; placebo: 71.4%; p=0.0480). For the Per Protocol (PP) population, Brilacidin-OM in the aggressive chemotherapy regimen similarly reduced the incidence of SOM by 80.3% as compared with placebo (Brilacidin: 14.3%; placebo: 72.7%; p=0.0249).”
http://www.ipharminc.com/press-release/2018/4...-mucositis
B-UP (Phase 2 POC)
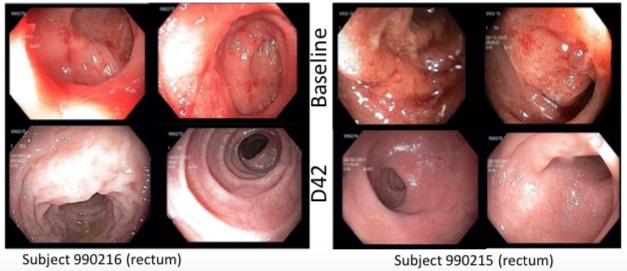
Before and after endoscopic images (Cohort B, 100 mg)
Topline Results
Rate of Clinical Remission (% of patients achieving)
- 60% Cohort A (3 of 5 patients)
- 67% Cohort B (4 of 6 patients)
- 75% Cohort C (3 of 4 patients)
(i) #Endoscopy subscore ≤ 1 (% of patients achieving)
- 80% Cohort A (4 of 5 patients)
- 67% Cohort B (4 of 6 patients)
- 75% Cohort C (3 of 4 patients)
(ii) Rectal Bleeding subscore of 0 (% of patients achieving)
- 80% Cohort A (4 of 5 patients)
- 100% Cohort B (6 of 6 patients)
- 100% Cohort C (4 of 4 patients)
(iii) Stool Frequency subscore, improvement or no change from baseline (% of patients achieving)
- 100% Cohort A (5 of 5 patients)
- 100% Cohort B (6 of 6 patients)
- 100% Cohort C (4 of 4 patients)
http://www.ipharminc.com/press-release/2017/7...brilacidin
B-ABSSSI (Phase 2b)
Topline Results
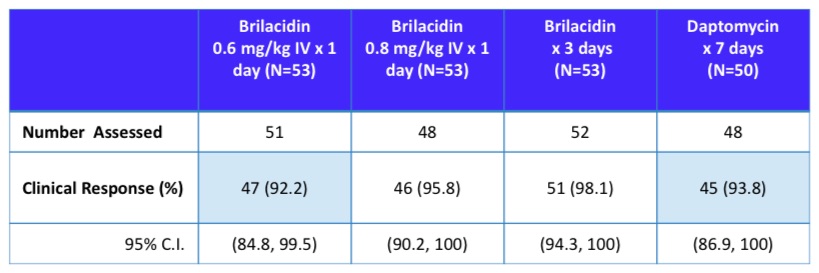
"In treated patients assessed at 48-72 hours, 47/51 (92.2%), 46/48 (95.8%), 51/52 (98.1%), and 45/48 (93.8%) achieved clinical success in the Brilacidin 0.6 mg/kg single-dose group, Brilacidin 0.8 mg/kg single-dose group, Brilacidin 1.2 mg/kg 3-day group, and daptomycin 7-day group, respectively."
"On December 22nd 2014, Cellceutix also reported positive results in the Microbiological Intent-to-Treat (MITT) population. This is an important population that includes patients with baseline cultures positive for common ABSSSI pathogens, such as Staphylococcus aureus, including Methicillin-Resistant Staphylococcus aureus (MRSA). In this population, Clinical Success rates at 48-72 hours were again very high (above 90% across all treatment groups) and again very similar (with overlapping 95% confidence intervals)."
http://www.ipharminc.com/press-release/2016/1...-of-absssi
Prurisol (Phase 2a)
Topline Results (200 mg)
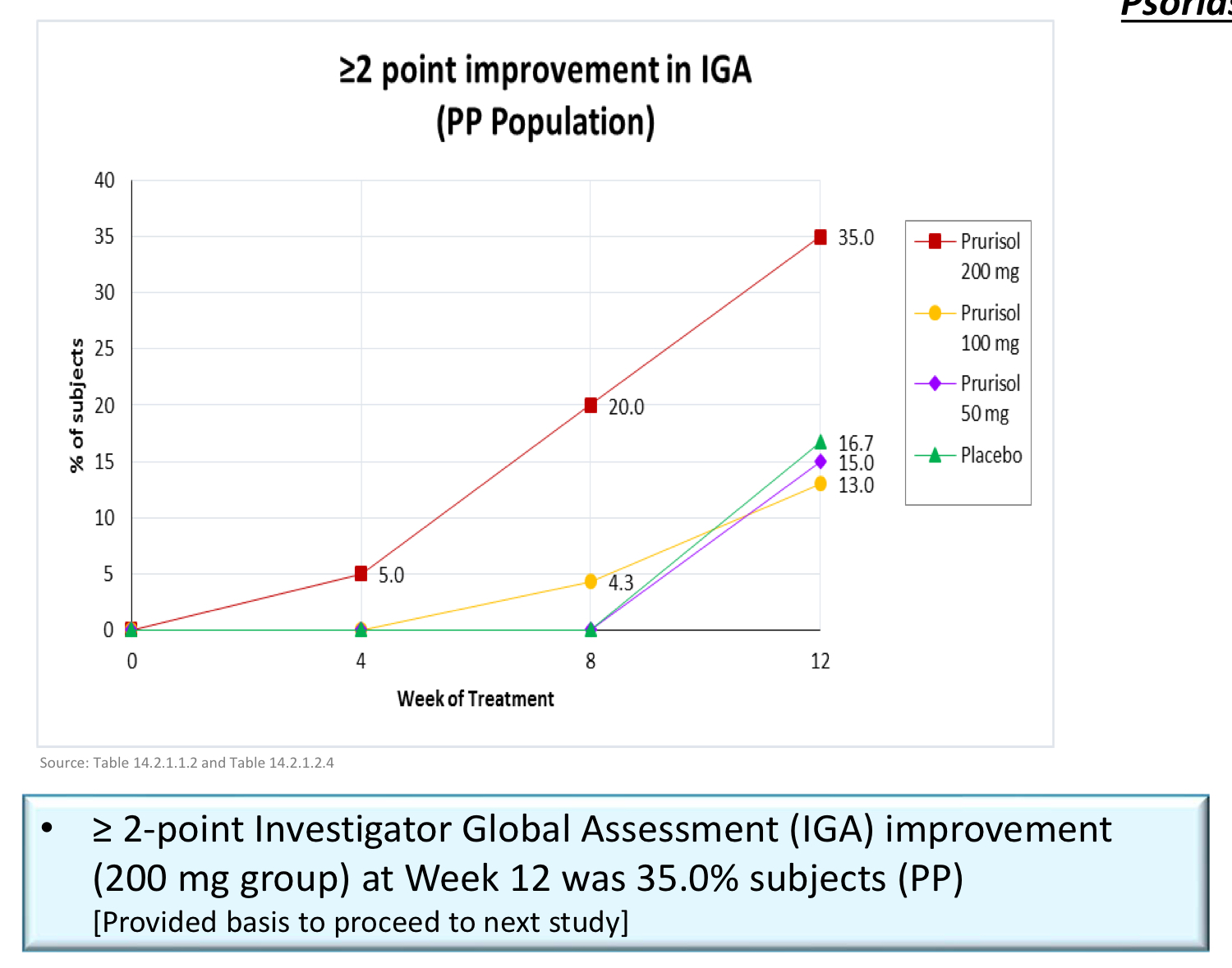
"Sub-population analyses further showed greater efficacy demonstrated in patients who had a baseline IGA score of 3 (“moderate”) as compared to those with a baseline score of 2 (“mild”). Some of these patients even experienced a 3-point reduction in their IGA score, going from “moderate” to “clear.” This suggests Prurisol may be more effective in treating moderate to severe psoriasis patients to a greater degree than those patients who exhibit less severe symptoms. In moderate to severe psoriasis studies, the placebo response also tends to be lower."
http://www.ipharminc.com/press-release/2016/1...y-endpoint
"Among patients participating in the study with the severest form of psoriasis, those having a baseline IGA score of 3 (“moderate”), the primary endpoint was met in 46.2% of patients who received Prurisol 200mg. This data was derived from analyses of all patients randomized across all 9 participating study sites."
"Additional preliminary data analyses of secondary endpoints show patients who received any dose of Prurisol, regardless of the treatment arm, had a 1-point improvement (using the IGA scoring system) at a higher rate than that of patients in the placebo arm. This is another clear indication of the drug’s efficacy."
http://www.ipharminc.com/press-release/2016/1...-psoriasis
K-OC (Phase 2a)
P53 Modulation
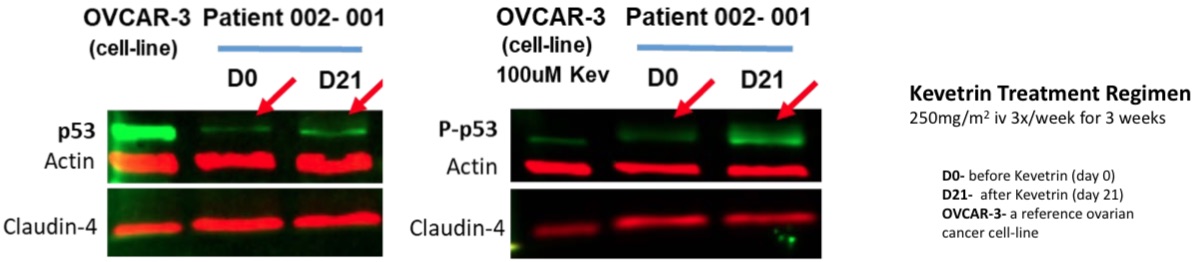
"Modulation of the p53 protein was observed in response to administration of Kevetrin. Pathways analyses also point to concomitant cell cycle modulation at the level of gene expression. Importantly, these data are the first to directly support, in ovarian cancer patient tumors, Kevetrin’s ability to affect p53 and associated molecular pathways—a central gene signaling network involved in regulating cell growth and the cell cycle, helping to prevent cancer."
"In more detail, preliminary analyses used Western Blots to assess relative levels of key proteins extracted from tumor biopsies before and after a series of nine Kevetrin infusions administered over three weeks. The level of phospho-p53, the activated form of the protein, in addition to the noted p53 modulation, was also seen to change in response to Kevetrin administration. These findings confirm in patient tumors Kevetrin-induced anti-cancer effects similar to those demonstrated (pdf) preclinically in ovarian cancer cell-lines. These new data reinforce prior clinical data, from the earlier concluded Phase 1 study of Kevetrin in advanced solid tumors (see NCT01664000), in which observations of p21 expression in peripheral blood monocytes supported p53 involvement in Kevetrin’s mechanism of action."
http://www.ipharminc.com/press-release/2017/1...ncer-trial
 (9)
(9) (0)
(0)Innovation Pharmaceuticals Inc (IPIX) Stock Research Links
