$GERN Geron Corporation (Part I): Elucidating The
Post# of 4531

Provided by: BioSci Capital Partners
Integrated biosci research, consultant
https://seekingalpha.com/article/4160973-gero...imetelstat
Summary
Lead molecule Imetelstat is potentially a novel cancer treatment gearing to inhibit the enzyme, telomerase.
Telomerase inhibition leads to shortening of the tail end of chromosomes for cancer's destruction.
Imetelstat is currently being investigated in two conditions (myelofibrosis and myelodysplastic syndrome).
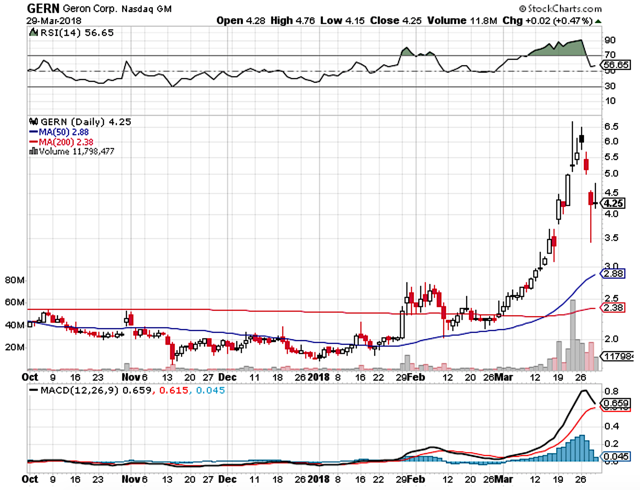
Geron Corporation (NASDAQ:GERN), a bioscience focusing on the development and commercialization of the telomerase inhibitor to manage various cancers, is a volatile stock with significant risks. Nevertheless, there are powerful underlying catalysts that have a highly favorable chance of catapulting the share northbound in the next few months. Of note, the stock appreciated over 81% in the past 52 week (to trade at $4.02 at the closing of the April 2nd trading session).
And, this is due to the clinical advancement of the lead molecule (Imetelstat) as well as the potential that Johnson & Johnson (NYSE:JNJ) will extend the collaborative partnership for the development of the said molecule. In this research, we’ll go over the underlying science of the telomerase inhibitor as well as the catalysts that can dictate the share price movement in the foreseeable future.
Fundamental Analysis
Based in Menlo Park, CA, Geron Corporation is focusing on the innovation and commercialization of a novel cancer treatment that leverages on the power to control the telomerase enzyme that, in and of itself, regulates the length of the human deoxyribonucleic acid (“DNA”). Lead molecule Imetelstat (a telomerase inhibitor) is currently being investigated in the Phase 2 (IMbark) study and Phase 2/3 (IMerge) trial as the potential treatment for myelofibrosis (“MF”) and myelodysplastic syndromes (“MDS”), respectively (as shown in figure 2).
Back in 2014, the stock took a nosedive, as the FDA put a clinical hold on the development of Imetelstat due to concerns re the reversibility of liver toxicities. The company submitted additional data which indicated that the toxicities were resolved. In response, the agency removed the hold and recommended the company study more serious conditions like MF.


In order to fully appreciate Imetelstat’s prospects, it is imperative to go over the underlying science of telomere. As a highly specialized area, the research on telomere and telomerase enabled us with a firm grasp on the roles of telomeres in normal cells. Interestingly, the early Geron collaborators (Elizabeth Blackburn, Carol Greider, and Jack Szostak) won the 2009 Nobel Prize in Physiology and Medicine for their work on telomere and telomerase.
Through their research, we now have a much better understanding of this field. Moreover, their research findings have far-reaching ramifications for cancer management that we’ll go over in details later. For now, let’s focus on the science of telomere and telomerase.
At the end of the chromosome (a structural unit that bundles up the DNA), there are regions having repeated genetic sequences known as telomeres. With every successive cellular division, the telomeres shortened to result in DNA loss. With enough shortening to reach a critical length, the cell eventually dies (or senesces).
Notably, the human physiology operates via homeostasis (a state of balance). And thus, telomerase adds in more DNA sequence at the telomeres to reduce genetic materials loss (as shown in figure 3). In adjusting the telomerase activity, the body can control the lifespan of various cells. And, this balance is critical for the normal cellular function as well as to prevent cancers.
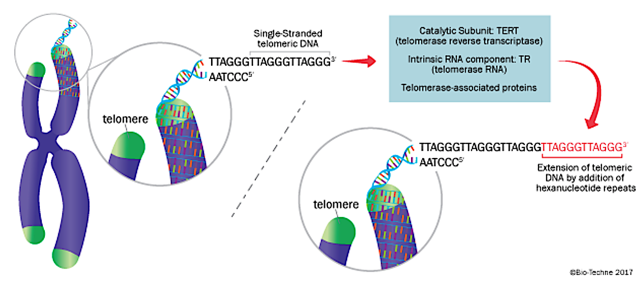
Telomerase in action - the synthesis and maintenance of telomeric DNA by adding TTAGGG repeat units onto the 3’ ends of existing single-stranded telomeric DNA. Telomerase contains protein components such as TERT, associated proteins, and an RNA subunit TR (Source: Rndsystem)
In most normal cells, telomerase is repressed, thereby allowing the telomere length to slowly decrease for the prevention of uncontrolled cellular division (and to enable a normal cellular death process coined apoptosis). For cells that divide rapidly (blood or gut lining), telomerase activity is upregulated in the progenitor (offspring) cells via homeostasis to maintain a normal telomere length. Else, the chromosomes of these cells would shorten too quickly to cause rapid cellular senescence. As daughter cells of those progenies become mature, telomerase activity is lessened to ensure that these cells do not live dangerously too long.
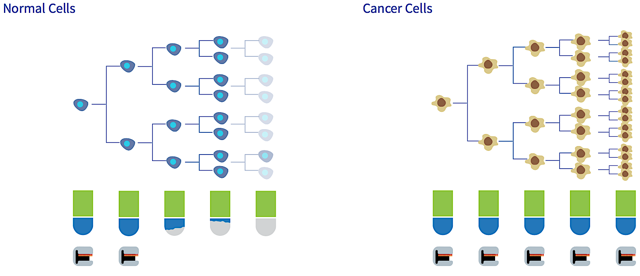
Interestingly, research found that there is an increased telomerase expression in 90% of biopsies taken from many different cancers (as illustrated in figure 4). Due to the heightened telomerase activity, cancer cells are able to maintain their chromosome length, thus subjecting them to uncontrolled division and immortality. As these rogue cells do not die off, the tumor grows larger to consummate most of the nutrients of normal cells while disrupting normal cellular processes.
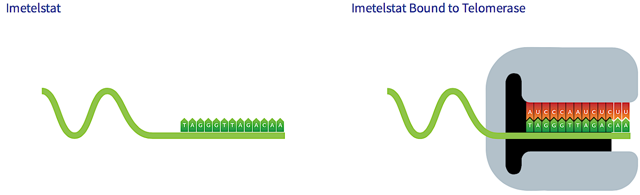
Let’s now look at how Imetelstat can regulate telomerase activity to deliver its therapeutic benefits. Formerly known as GRN163L, Imetelstat is a medicine designed for infusion. It is a 13-mer N3’-P5’ thio-phosphoramidate (“NPS”) oligonucleotide that is covalently linked to 5’ palmitoyl lipid group at the C16 position. Imetelstat “competitively” binds to telomeres to prevent telomerase from attaching to the same target. By inhibiting telomerase via Imetelstat, the loss of genetic materials is expedited to cause cellular deaths (as shown in figure 5).
As alluded, telomerase inhibition is highly efficacious against cells that divide rapidly. This includes not only cancer cells but also hair cells as well as those lining the guts. The cellular destruction (be it via the standard of care chemotherapy or telomerase inhibition) will lead to side effects. As the intestinal lining is sloughed off because these cells divide quickly (and are killed), the patients to experience nausea, vomiting, potentially bleeding, and appetite loss.
Another example is that a drug that attacks rapidly dividing hair cells (i.e. chemotherapy) causes patients to become bald. Be that as it may, the mechanism of action of Imetelstat gives it key advantages as well as enables it to bypass those limitations faced by chemotherapy.
First, the backbone of Imetelstat enabled it to survive the enzymatic degradation by cellular nucleases. That backbone also improves binding affinity to specific targets. Second, the palmitoyl region of Imetelstat enabled it to cross the cell membrane to go to the cell nucleus (where the chromosomes reside to exert its therapeutic effects). As a result, the lipid permeability improves the pharmacokinetic as well as pharmacodynamic properties.
Third, Imetelstat preferably accumulates in cells of tissues with high-fat contents (i.e. bone marrow, spleen, and liver) due to the palmitoyl lipid side chain. The binding preference enabled a long residence time in these tissues. This is highly significant, as it allowed the drug to target specific cancers where the cellular progenitors resides in those mentioned tissue like blood cancers.
Of note, blood cells are made in the bone marrow where there is a high-fat content. And, it is the target specificity that helped to minimize the adverse effects against other rapidly dividing cells like hair cells or those of intestinal linings (both of which lead to significant adverse effects and are notable limitations of conventional chemotherapy).
Fourth, the competitive binding as mentioned means that the attachment is not permanent (Imetelstat simply outcompetes telomerase in the reversible binding to telomeres). Therefore, the normal cellular machinery that make proteins (translation) won’t be disrupted. Hence, that mechanism of action enabled the minimizing of adverse effects.
Imetelstat is a highly interesting drug because it has the tissue preference for the bone marrow. As mentioned, the lipid linker enabled the tissue specificity for those with high fats (the bone marrow), which limits the adverse effects and improves efficacy (over conventional chemotherapy). In the marrow, there are many different types of blood cells. These cells originated from different progenitor lineages that, in and of itself, came from the common stem cells.
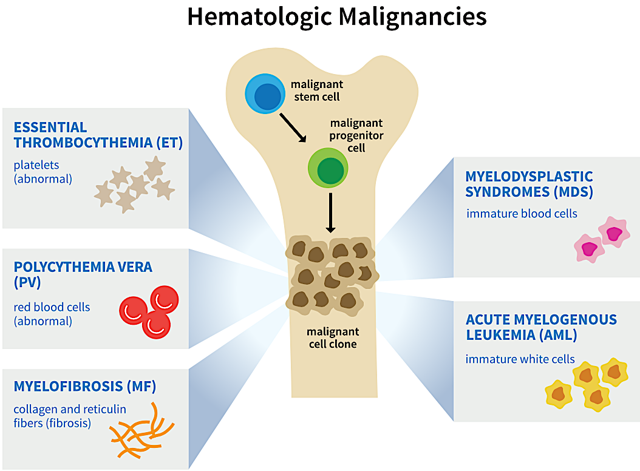
Red blood cells (“RBCs”) are responsible for the transport of oxygen and carbon dioxide. White blood cells (“WBCs”) are in charge of defending against cancer and foreign invaders. Platelets are used for wound clotting. Blood cancers are classified based on the cell lineages that those cancers originated from. Different blood cancers and diseases are due to malignancy of different progenitor (precursor) cells (as depicted in figure 6).
Having defects in the lymphoid lineage (WBCs like B or T cells), some lymphoblastic or lymphocytic cancers include acute lymphoblastic leukemia, chronic lymphocytic leukemia, lymphomas and multiple myeloma. Conversely, myelogenous or myeloid cancers took root in the malignant precursors to RBCs, platelets, and WBCs (granulocytes).
Examples of myelogenous cancers are acute myelogenous leukemia (“AML”), chronic myelogenous leukemia, myelodysplastic syndromes (“MDS”) and the myeloproliferative neoplasms, such as essential thrombocythemia (“ET”), polycythemia vera, and myelofibrosis (“MF”). Of note, we are most interested in MF and MDS, as Imetelstat is currently being investigated for those conditions.
The early clinical outcomes were highly encouraging with the robust response rates in all three franchises (ET, MF, and MDS). As mentioned, the FDA put a clinical hold on ET but subsequently removed it, as the liver fibrosis was reversible. Despite that the data was from the early study, it is most likely the case that the agency believes in the therapeutic efficacy of Imetelstat to recommend its continuing innovation in the MF and MDS.
The lift on clinical hold is significant because, even with the potential adverse effects, the drug can still gain the FDA approval when it can demonstrate that benefits outweigh the risks. Not all drugs are free from adverse events, and the clinical decision to prescribe the drug is all dependent on the benefits versus risks. Facing the potential adverse events, if the life-threatening conditions like MF and MDS do not have many therapeutic options, it is most likely that it can gain approval.
Final Remarks
By suppressing the natural enzyme telomerase, the drug has been shown in early clinical data to cause significant cancer remissions. Regardless of the early clinical setbacks, Geron rebounded and is currently powering two highly promising franchises (MF and MDS). Safety concerns have been overly pessimistic while efficacy results have been stellar, which caused the recent share price recession for this highly volatile stock. The main investment risk for Geron is whether Imetelstat can deliver robust results for both MDS and myelofibrosis.
Moreover, the other risk is if Johnson & Johnson will continue their partnership in Q3. Due to its binary nature, any negative event for Geron can cause the stock to tumble over 80 rather than the usual 50% mark. But this stock can be a multi-bagger if the binary catalysts prove positive over time. Of note, there is a higher-level intelligence published in advanced and exclusively for subscribers of Integrated BioSci Investing. In the said research, we elucidated this thesis in far greater details with specific recommendations.
Author’s Notes: We’re honored that you took the time to read our market intelligence. Founded by Dr. Hung Tran, MD, MS, CNPR, (in collaborations with Analyst Vu, and other PhDs), Integrated BioSci Investing (“IBI”) is delivering stellar returns. To name a few, Nektar, Spectrum, Atara, and Kite procured over 424%, 97%, 163%, and 83% profits, respectively. Our secret sauce is extreme due diligence with expert data analysis. The service features a once-weekly exclusive Alpha-Intelligence article, daily analysis/consulting, and model portfolios. Subscribe to our marketplace now to lock in the current price and save money for the future.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: I like to inform our readers of Seeking Alpha's recent policy change, in which the company implemented the paywall (not only to my articles but to all articles that are published over 10-day). This is in place, as the company is, after all, a business. And, the revenues from ads are not adequate to support the high-quality research that the company is providing. If you are a REAL TIME FOLLOWER, you will be notified immediately of our new research for you to continue to benefit from our due diligence. You can also gain access to all of my old articles and much more by taking the 2-week FREE trial of my marketplace, Integrated BioSci Investing.
 (0)
(0) (0)
(0)