$ALRT: FDA website update to show 510(k) Premarket
Post# of 110700

PremarketNotification(1).jpg)
https://www.accessdata.fda.gov/scripts/cdrh/c...ID=K163664
ALR Technologies Inc (OTCMKTS:ALRT) Takes Traders On A Wild Ride
https://insiderfinancial.com/alr-technologies...da/172062/
CERTAIN AMOUNT OF RISK INVOLVED: 8K FILED 09/22/17
ITEM 7.01 REGULATION FD DISCLOSURE.
510(K) Clearance
On September 19, 2017 the Company announced that it has received 510(k) clearance from the United States Food and Drug Administration for its insulin dose adjustment feature of the ALR Technologies Inc. Diabetes Managements System (also referred to as the “ALR Health-e-Connect System”).
Deficient Reporting Status
The Company has received notice from the Securities and Exchange Commission (the “SEC”) that the Company is not in compliance with its reporting requirements under the Securities Exchange Act (the “Act”). If the Company does not file all required reports, the Company may be subject to an administrative proceeding to revoke its registration under the Act. The notice also stated that the Company stock may be subject to a trading suspension by the SEC pursuant to the Act. At this time, the Company does not have the resources to file all required reports to bring the Company into compliance, and to continue filing the required reports to maintain compliance.
http://www.otcmarkets.com/edgar/GetFilingHtml...D=12290355
Consider that this ticker ran over 12,000% in a single day when this news was announced (before FDA website update). A/S=100M with CEO and wife owning 97% on a fully diluted basis. They have worked on this device over 10 years and poured a lot of their own money into it; are we to believe that they will allow the stock to be suspended due to filing deficiencies? Additionally, they say in emails posted in various places (authenticity subject to debate) that there is more news coming within 45 days.
EMAILS (decide for yourself if they are authentic)
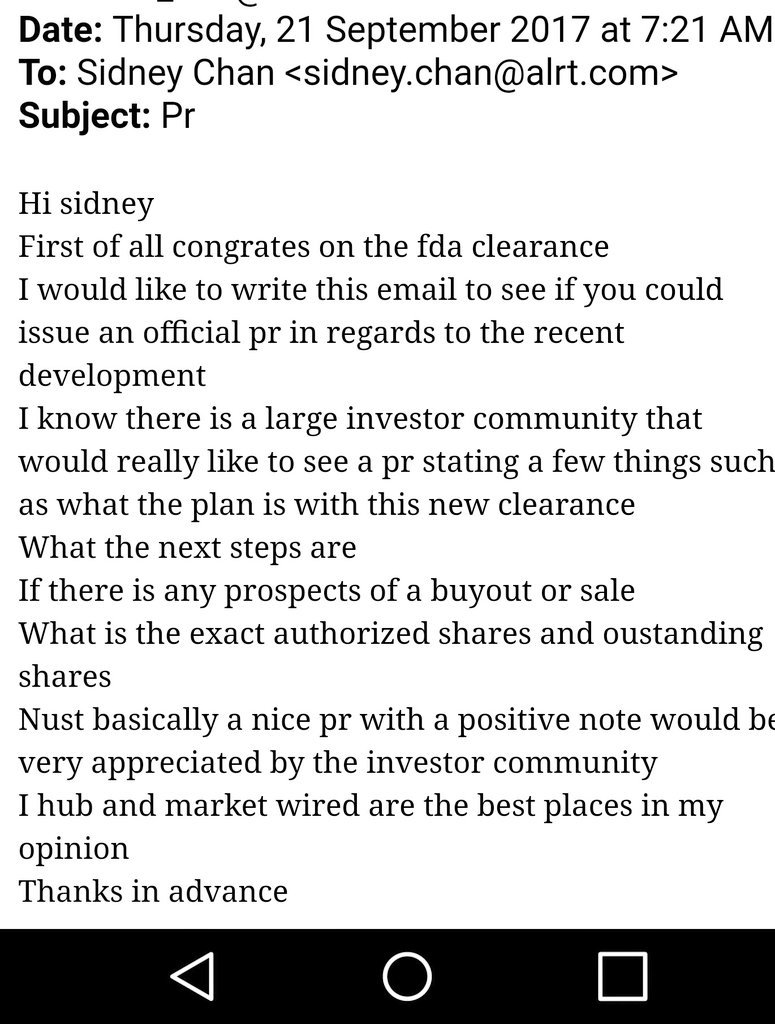
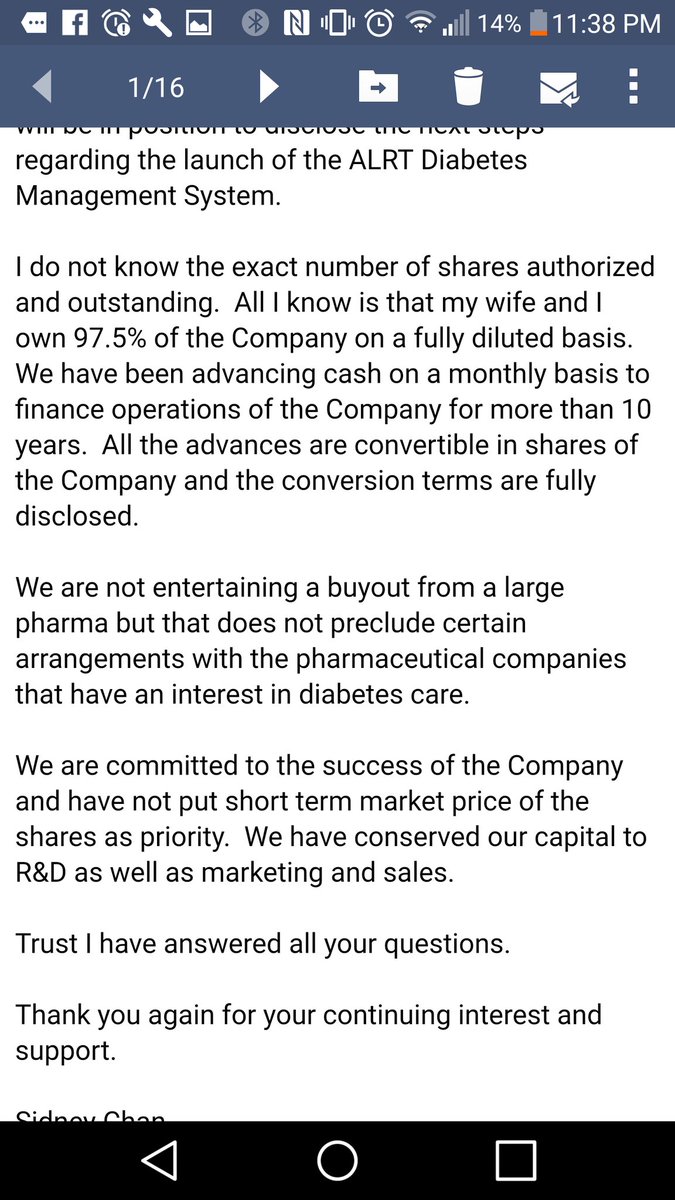
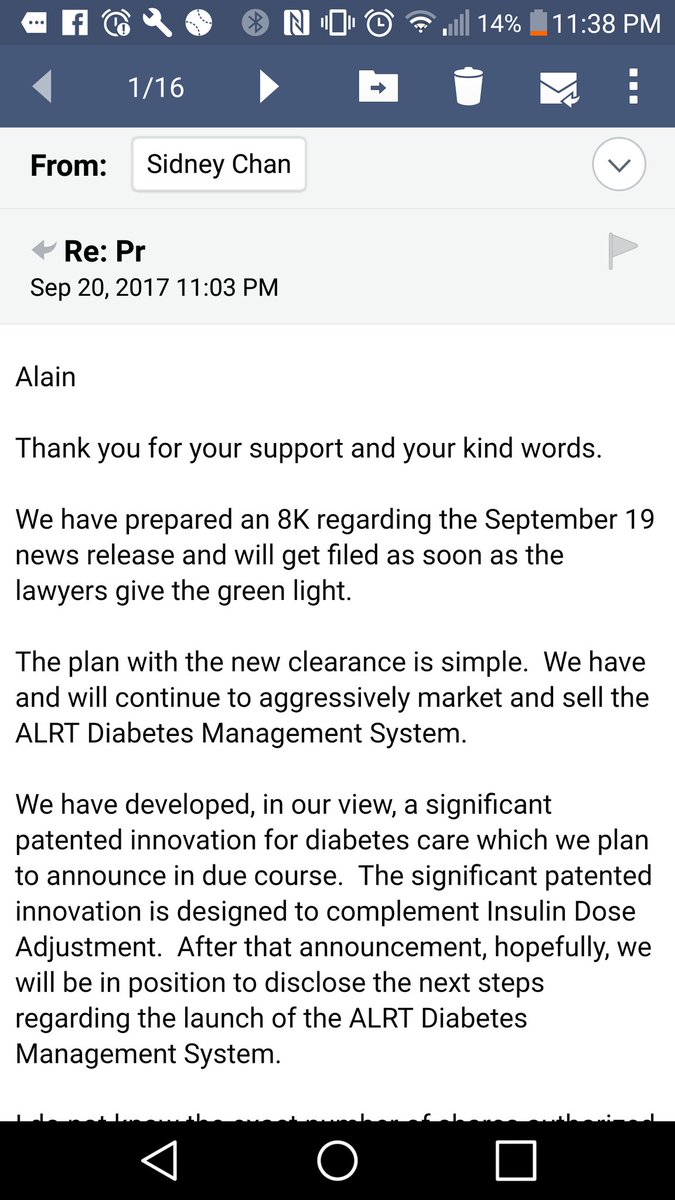
One must consider the SUBSTANTIAL risk/reward in this potential trade.
 (0)
(0) (0)
(0)