on CLSN...........................................
Post# of 226

on CLSN................................................
Celsion's Phase III Blockbuster Data Revealed And It's Been Right Underneath Your Nose
"...one of the most important new drugs in a generation"
"...perhaps a blockbuster by anybody's definition"
-- Celsion ( CLSN ) CEO Michael H. Tardugno in a recent presentation .
There's been a lot of scientific and medical debate from bulls and bears as to why the Celsion ThermoDox drug in Phase III results should work or not. Forget "should" -- there's already enough data publicly revealed to prove mathematically that ThermoDox already has worked. I'll explain.
There have been many great articles already recently that dive into the scientific and medical background about this study. I will direct you to my personal two favorite articles ( here and here ) which I agree with its contents and leave it at that. This article's intent is to move on from theory and analyze what is already a historically revealed fact.
Celsion is literally any day away from its top-line release of its Phase III study, called the HEAT trial, designed to show that ThermoDox + RFA treatment improves the time to progression of HCC liver cancer by at least 33% for patients with large tumors versus RFA alone. For this trial, a Progression-Free Survival (PFS) event is usually when either a patient's treated tumor has a recurrence (a local recurrence) or when a new tumor emerges outside of the area of the treated tumor (a distant recurrence). The longer the time this takes, the better it is for the patient, and the more effective ThermoDox is.
Let me make this clear: this isn't an article about "why" or "should" ThermoDox will beat the control arm, but rather a mathematical proof based on valuable data already in front of you. As a CLSN investor, this is what is relevant to me. And to me, the math shows it's already a statistical fact that ThermoDox must be working. Using nothing but publicly-released data that's been sitting right underneath your nose, my calculations will show:
ThermoDox has slowed the progression of the disease over the control arm by at least 144% (probably much more)
What we know:
(1) We now have enough data points to know how many patients enrolled between various dates that allows us to calculate approximately how long each of the patients have been in the trial.
(2) We know how many of them have shown FDA-defined progression in their liver cancer.
(3) We know how many statistically should have shown FDA-progression in their liver cancer if ThermoDox didn't work.
(4) From a table of this data, we can fairly easily calculate if ThermoDox works or not.
Clearly ThermoDox works. It has to, and the math proves it.
THE PROOF:
Two most likely causes cited by the FDA that constitute a progression-free survival event are quoted as "local recurrence" or "Any new distant intrahepatic HCC tumor."
Combing the enrollment data with known averages for local recurrence and distant recurrence for RFA-only, it's very easy to then calculate what the trial population would be expected to show for RFA-only (if ThermoDox had no effect). This data, results of data into excel-driven formulas, and conclusions are all below and haven't been revealed or even hinted at in any other article that I can find. CLSN insiders I'm sure have done similar calculations, hence they're buying in the open market and even exercising options ahead of phase III release data without selling the stock. They feel they can't lose.
| PR Date | Total | Incre. | Months to PFS 380 | Total | Prob. Of local | Prob. Of distant | Expected |
| Source | Enrollment | Enrollment | (est. 11/1/12) | Months | tumor recurr.-free | tumor recurr.-free | PFS Events |
| 09/30/08 | 20 | 20 | 36 | 720 | 0.50 | 0.25 | 17.50 |
| 08/05/09 | 151 | 131 | 36 | 4716 | 0.50 | 0.25 | 114.63 |
| 11/10/09 | 196 | 45 | 36 | 1620 | 0.50 | 0.25 | 39.38 |
| 01/20/10 | 250 | 54 | 34 | 1836 | 0.50 | 0.29 | 46.13 |
| 05/04/10 | 331 | 81 | 30 | 2430 | 0.50 | 0.38 | 65.81 |
| 08/03/10 | 405 | 74 | 27 | 1998 | 0.50 | 0.44 | 57.81 |
| 09/30/10 | 450 | 45 | 25 | 1125 | 0.50 | 0.48 | 34.22 |
| 11/11/10 | 474 | 24 | 24 | 576 | 0.50 | 0.50 | 18.00 |
| 02/11/11 | 516 | 42 | 21 | 882 | 0.50 | 0.56 | 30.19 |
| 03/25/11 | 534 | 18 | 19 | 342 | 0.50 | 0.60 | 12.56 |
| 05/12/11 | 558 | 24 | 18 | 432 | 0.50 | 0.63 | 16.50 |
| 07/11/11 | 588 | 30 | 16 | 480 | 0.50 | 0.67 | 20.00 |
| 08/03/11 | 600 | 12 | 15 | 180 | 0.50 | 0.69 | 7.88 |
| 05/30/12 | 700 | 100 | 6 | 600 | 0.75 | 0.88 | 34.38 |
| TOTAL | 700 | 343 | 17937 | 515 | |||
| Avg. | 25.62 |
OBSERVATIONS AND CALCULATIONS:
(1) Using the enrollment data, the reflected mean and median time patients have been in the trial is at least 25.62 months and 27 months respectively.
(2) Based on mean and median, 75% to 80% of the trial population should have reached PFS event status.
(3) But only 54.2% (380 out of 701) reached it.
(4) Using the above enrollment data and breaking down the expectations into the 14 mini groups yields a more accurate expectation of at least 515 or 73.47%.
(5) Using the 73.47% on the control arm of 350 yields, 257 patients are expected to have reached a PFS event.
(6) 380 PFS total minus the 257 from the control arm leaves only 123 expected ThemoDox patients who reached a PFS event.
(7) 257 control arm patients is 109% (or more) than in the ThermoDox arm expected to have had PFS event.
(8) 35.1% (or less) patients who received ThermoDox had a PFS event, with the mean/median population nearly halfway to the 5-year mark, which also means 64.9% still not having yet reached PFS.
(9) The median expected PFS of the control group is 10.5 months. The median expected PFS of the ThermoDox group is undetermined since the ThermoDox patients have to even reach a 50% median yet after over 2 years! (very good news).
(10) With an average of 25.6 months and still no median yet, ThermoDox patients are already showing an expected at least 144% improvement in PFS, and the 144% figure will rise significantly by some not yet known amount as the trial progresses.
(11) Some people define "cure" or "curative" as patients who make it 5 years without any disease progression. With 64.9% making through the halfway mark, at which point the charts get more "flat," a significant portion of the ThermoDox population are expected to reach 5 years without showing any recurrence of cancer progression.
Conclusion: The phase III data will show that ThermoDox works for the majority of patients showing a very large improvement in PFS and even a cure for a good number of patients.
RELEVANT CHARTS:
Local :
"but beyond 2 cm recurrence rate in the zone of ablation is over 50% and increases rapidly with the size of the tumor" (CLSN's Phase III is for 3 cm or greater)
To be ultra-conservative, I used 50% at 12 months.
(click to enlarge) 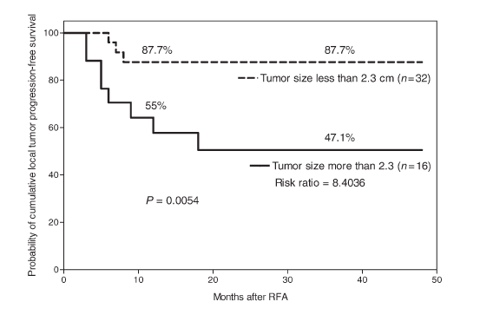
source : Japanese Journal of Clinical Oncology
Distant :
53.7% at 24 months, in line with many other studies cited in this PDF.
I used 50% to be conservative and for simplicity.
(click to enlarge) 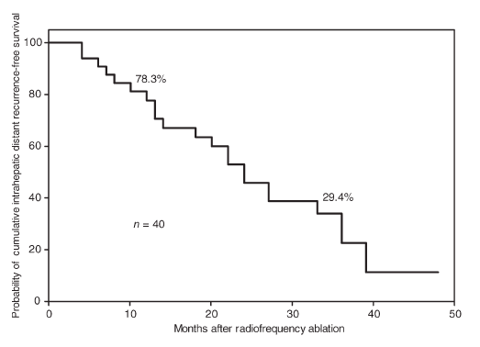
source : Japanese Journal of Clinical Oncology
With all this in mind, there are several risks for CLSN stock:
(1) I could be missing something or my data is wrong. It happens sometimes, despite having several check on my work on this.
(2) There could be some unknown, unexpected side effects that are unacceptable and only came to be realized with final analysis of the data
(3) I could be correct in my analysis, but the stock could have already priced in a positive data reaction in the short term and the stock could sell off on the news (buy the rumor, sell the fact).
(4) New more superior competition could emerge.
(5) The company itself could stumble, make an error, something could happen to a key man in the company, there could some sort of internal power struggle drama within the company that develops, etc. Things such as this happen sometimes, especially with tiny companies.
Mathematically speaking, though, ThermoDox has to work. There's no other plausible explanation, and I believe not only with the world realizing this when the Phase III data is released, but the world should have seen it coming from a mile away. After all, there's been enough as I have revealed that's been sitting right underneath everybody's nose already.
 (0)
(0) (0)
(0)