7 Companies Fighting To Cure Cancer December
Post# of 72451

7 Companies Fighting To Cure Cancer
In America, cancer is the second leading cause of death, after heart disease. The disease kills an estimated 567,000 people each year. Every year, people rally around trying to find a cure. They raise money through special events, fundraisers, phone campaigns, and marathons. Many companies in the biotechnology space have invested millions of dollars to try and find a cure. With so many different types of cancer, companies typically focus on treating one and then potentially applying their technology to other forms. Below are 7 promising companies that have upcoming catalysts for their cancer drug. I focused on companies that are developing primary treatments for forms of cancer that don't currently have a solution.
Success Story: Pharmacyclics, Inc. ( PCYC )
Pharmacyclics is a clinical-stage biopharmaceutical company focused on developing and commercializing small-molecule drugs for the treatment of cancer and immune mediated diseases. This company is a great example of a company that is able to apply their methodology to different forms of cancer.
Ibrutinib is a product from PCYC that is used to treat chronic lymphocytic leukemia, commonly known as CLL. The goal is to be able to use the drug without chemotherapy, as it will reduce toxicity levels and make the treatment more tolerable for all patients. The treatment demonstrated impressive numbers with 71% of untreated patients receiving a complete or partial response. The same response was noted in 50% of the high risk group.
The company has taken its impressive treatment and currently has several ongoing trials to attempt to treat other forms of cancer. Pharmacyclics has several trials evaluating ibrutinib in the treatment of CLL, SLL, Mantle cell lymphoma (a type of Non-Hodgkin lymphoma), and other blood cancers.
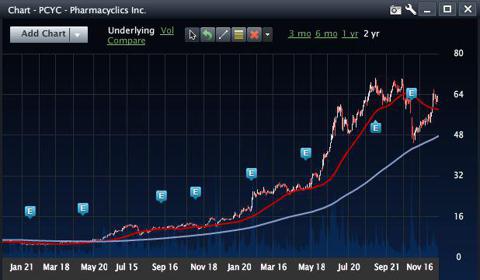
The stock chart below indicates what happens when a company can apply its science to treat other illnesses, especially cancer.
(1) Celsion Corporation ( CLSN )
Company Profile: Celsion is an oncology drug development corporation focused on the development and commercialization of treatments for those suffering from different forms of cancer.
Type of Cancer Being Treated: Liver Cancer
Upcoming Catalyst:
- The drug facing Phase III trial results is ThermoDox, used in the prevention of primary liver cancer. This would become the primary treatment for liver cancer in the world.
- Trial Results: By the end of January 30th, 2013.
Why Investors Should Care: The company has announced that it will release its Phase III ThermoDox results by the end of January. This is a top-line release of a multinational, double-blind, placebo-controlled study of primary liver cancer. The CEO has stated that this drug has the potential to generate yearly revenues of $1 billion. If successful, it would become the primary treatment for primary liver cancer around the world. With the current market capitalization sitting at roughly $260 million, investors have an enormous opportunity to realize gains. Additionally, if successful, the treatment has the potential to be used for other types of cancer as well.
Risks: With any trial release, there is always the strong possibility of failure. Should this occur, it is likely that the stock would nosedive to as low as $1 per share, possibly even lower. This is truly a make or break announcement for the company. Additionally, the company could announce it has succeeded in the trial but the street may not think the numbers are as promising as they had hoped. This could also result in a significant share price reduction. There is also potential competition concerns from Pfizer ( PFE ), who is very early on in the process of developing a competing drug for liver cancer.
(2) Threshold Pharmaceuticals ( THLD )
Company Profile: Threshold Pharmaceuticals is a development stage company, focused on the discovery and development of drugs for the treatment of various forms of cancer. Its primary clinical development product is TH-302, which has already successfully passed Phase I and Phase II trials .
Type of Cancer Being Treated: Pancreatic Cancer
Upcoming Catalyst:
- The drug facing Phase III trial results is TH-302, used to treat pancreatic cancer.
- Trial Results: By the Fall of 2013
Why Investors Should Care: Threshold has a lot of science behind its most notable drug, TH-302. In addition to becoming a primary treatment for pancreatic cancer, TH-302 had a positive Phase II release for the treatment of soft tissue sarcoma. There are potentially a lot of other applications of Th-302 as well. And with the backing of Merck GAaA ( MKGAF.PK ), the company shouldn't have to complete more fundraising (always a concern for existing shareholders).
Risks: The main risk for the company, aside from the possibility of bad data, is competition from Celgene (CLGN). Celgene's drug "Abraxane," used in the treatment of pancreatic cancer, recently release successful Phase II I results. This puts it ahead of Threshold in terms of being able to reach the market first.
(3) Dendreon Corporation ( DNDN )
Company Profile: Dendreon is a biotechnology company focused on the discovery, development, and commercialization of therapeutics that may improve cancer treatment options for patients. Its most notable drug is Provenge, used in the treatment of prostate cancer.
Type of Cancer Being Treated: Prostate Cancer
Upcoming Catalyst:
- The drug Provenge, used in the treatment of prostate cancer, is facing European Approval.
- Decision due in early 2013
Why Investors Should Care: Dendreon has certainly had its fair share of up and downs over the past 2 years. However, there is reason to be optimistic and that optimism centers around the company's Phase II study. It combined Provenge and two other drugs that have been shown to shrink tumors. The primary goal of the trial is to test the effectiveness of Provenge. Studies that were completed in animals demonstrated that the combination of Provenge and the other two drugs increased survival and tumor regression in more than 50% of mice.
Risks: Three potential concerns are dilution, competition, and cost. Dendreon will need to finance these additional trials and that will most likely come through additional secondary offerings, which will be at the expense of existing shareholders. Additionally, the company faces competition from Johnson & Johnson's competing product called Zytiga. This drug showed that men lived twice as long before their cancer worsened. And lastly, Zytiga only costs $5,500 a month, whereas Dendreon's Provenge costs a total of $93,000.
(4) Cellceutix Corporation ( CTIX.OB )
Company Profile: Cellceutix is a development stage biopharmaceutical company, engaged in the discovery and development of small molecule drugs to treat diseases primarily in the areas of difficult to treat cancers, psoriasis, autism, and inflammatory disease. The full company's pipeline includes eight drugs, with the most notable being Kevetrin and Prurisol.
Type of Cancer Being Treated: Solid Tumors
Upcoming Catalyst:
- The drug facing Phase I results is Kevetrin, used in the treatment of solid tumors.
- Results: Due out anytime in early 2013
Why Investors Should Care: Kevetrin is currently in Phase I trials and has shown the ability to be effective against solid tumors in pre-clinical studies. The drug also demonstrated potent anticancer activity against every type of cancer that was tested including lung, breast, colon, prostate, squamous cell carcinoma, and brain tumors. Kevetrin works to active the p53 gene, known as the "Guardian Angel Gene" because of its crucial role in controlling cell mutations. It is also the most commonly disrupted gene in cancer. Currently, there are more than 10 million people with tumors that contain inactivated p53. The market is huge!
Risks: There are several risks to Cellceutix. It has not completed its Phase I trials yet, so the company is still considered extremely early on in the process. The chances of failure are high. Additionally, the company will face stiff competition in several lines of cancer treatments. Lastly, the stock trades on the OTCBB where the lack of stringent rules has cost investors a lot of money over the years.
(5) Johnson & Johnson ( JNJ )
Company Profile: Johnson & Johnson engages in the research and development, manufacture, and sale of various products in the health care field worldwide. It offers products for use in baby care, skin care, oral care, wound care, and women's health fields. The company also has a pharmaceutical division which provides products in the areas of anti-infection, antipsychotic, contraceptive, dermatology, hematology, immunology, neurology, oncology, and pain management.
Type of Cancer Being Treated: Prostate Cancer
Upcoming Catalyst:
- The drug facing an approval decision is Zytiga, used in the treatment of prostate cancer.
- Approval Decision Date: April 15, 2013
Why Investors Should Care: Zytiga is an interesting product because it is a direct competitor of Dendreon's Provenge. Zytiga is a fraction of the cost that Provenge is, which has the potential to catapult Zytiga to become the primary treatment for prostate cancer.
Risks: Johnson & Johnson is one of the world's largest pharmaceutical companies in the world and is thus not dependent on a single drug. That being said, the stock is within a 52 week high and there are some who believe that approval of Zytiga is already built into the share price. Should that approval not occur, there could be a bit of a selloff. Additionally, companies like Johnson & Johnson are always in search of new treatments and in order to justify their market capitalizations, new developments must occur routinely.
(6) Celldex Therapeutics ( CLDX )
Company Profile: Celldex Therapeutics is a biopharmaceutical company focused on the development, manufacture, and commercialization of novel therapeutics for human healthcare. The company markets Rotarix to treat rotavirus infection. Its lead drug candidate is CDX-110, which is an immunotherapeutic vaccine. It is currently in Phase III clinical trials to target the tumor-specific molecule, epidermal growth factor receptor variant III. Its other lead drug candidate is CDX-011 which is the focus of this article. This drug is an antibody drug conjugate used to treat metastatic breast cancer and melanoma indication.
Type of Cancer Being Treated: Breast Cancer
Upcoming Catalyst:
- The drug facing Phase III results is CDX-101, used in the treatment of breast cancer.
- Results Due by end of 2013
Why Investors Should Care: A few days ago, the company announced its Phase II results. The results showed that the drug delayed tumor growth and prolonged survival in patients with advanced and aggressive form of breast cancer compared to single-agent chemotherapy. CDX-011 is a monoclonal antibody drug conjugate. The antibody portion connects with cancer cells containing the protein known as GPNMB. This protein has been shown to correlate with poorer outcomes in breast cancer patients. When the attachment occurs, CDX-011 releases a toxic chemotherapy payload. This drug conjugate was licensed from Seattle Genetics ( SGEN ) and is the same one used in the newly-approved lymphoma drug Adcetris. Celldex designed the Phase II study to test the theory that CDX-011 would work better in breast cancer patients with tumors containing high levels of GPNMB protein. The 120 patients enrolled in the study were screened to make sure they all had at least some tumor cells that contained GPNMB. The median overall survival almost doubled in the CDX-011 treated patients to 10 months versus 5.5 months for patients in the control arm.
Risks: Although the results and numbers are promising, one concern is that this was a small study. With only 120 patients enrolled (clearly a small sample size), Celldex will have to complete an additional study with a larger base and prove that the same results can occur.
(7) ImmunoCellular Therapeutics ( IMUC )
Company Profile: ImmunoCellular is a development-stage company seeking to develop and commercialize new therapeutics to fight cancer using the immune system. The product candidate includes cellular immunotherapies targeting cancer and cancer stem cell antigens, peptide based immunotherapies targeting cancer stem cells, and monoclonal antibodies to diagnose and treat cancers such as glioblastoma multiforme, ovarian cancer, small-cell lung cancer and pancreatic cancer.
Type of Cancer Being Treated: Brain Cancer
Upcoming Catalyst:
- The drug facing Phase II results is ICT-107, used in the treatment of brain cancer.
- Results expected in early 2013
Why Investors Should Care: During the Phase I trial, the company announced overall survival rates of 50% after four years, and 38% of patients remained progression free for 48 to 66 months. Historically, with this type of cancer, patients have an overall survivor rate of 12.1% after four years and a progression free survival of 5.6% after 48 months with standard of care. However, it was a small trial. So the larger Phase II trial will really tell investors what they can expect from this company and ICT-107.
Risks: Although Phase I trial results were impressive, it was only a single-arm study so there was no comparison arm. That makes it difficult for investors to really dig into the study. Additionally, brain cancer is one of the toughest forms of cancer to cute. It makes failure not only possible but probable.
Conclusion
Within this list, investors must decide which companies are the most compelling. For me, the most compelling include Celsion and Cellceutix. Celsion would become the only known treatment for the very hard to treat liver cancer. Cellceutix has shown that its science may be applied to seven different forms of cancer. These are my two largest holdings and I feel the future is extremely promising for them.
 (0)
(0) (0)
(0)