the run continues but still terrible underpriced
Post# of 83
Market Cap laughable $42 Million / Monster Pipeline / Lots of News around the corner = LIFETIMER OPP HERE
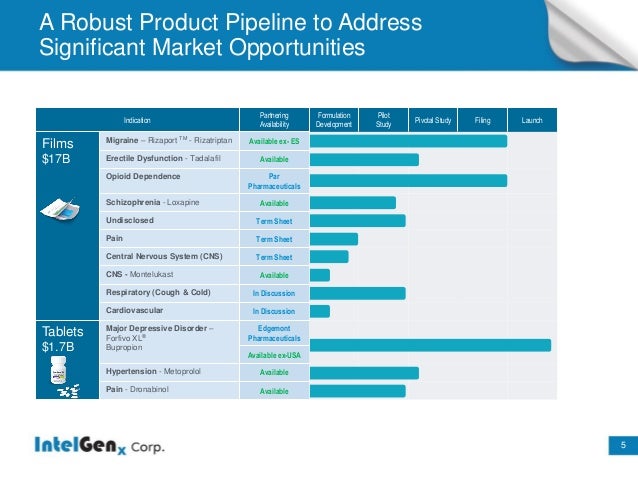
Upcoming Milestones :
Big Partnership imminent :
•Announced a development and commercialization term sheet with a global pharmaceutical company for up to three products. If entered into, IntelGenx expects the definitive agreement to be finalized in the second quarter of 2016
Product :Rizaport (Migraine)
•European Mktg Approval–November 2015
•Planned USA submission to FDA Q4/2016
•Expected USA launch Q2/2017
Product :Tadalafil (Erectile Dysfunction) a better Oral thin-film version of Blockbuster Drug Cialis
•505(b)(2) USA NDA submission in Q4/2016
•Expected USA launch Q4/2017
Product :Indicated for Opioid Dependence
•Awaiting FDA approval
•According to IMS data, the oral film market for opioid dependence was worth more than $1.4B US in 2014.
IntelGenx Corp., today announced the recent initiation of a phase 1 clinical trial of montelukast, a unique drug repurposing opportunity for the treatment of degenerative diseases of the brain, such as: mild cognitive impairment and Alzheimers disease, the most prominent form of dementia. IntelGenx expects results from the phase 1 trial to be available in September 2016.
 (0)
(0) (0)
(0)