SLNCF News Alert Silence Therapeutics (SLNCF) 3.7200 02/15/2015
Post# of 64074
Liver Fibrosis - Pipeline Review, H1 2014
M2 - Thu Dec 18, 4:04AM CST
Research and Markets (http://www.researchandmarkets.com/research/wmnbdp/liver_fibrosis) has announced the addition of the "Liver Fibrosis - Pipeline Review, H1 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Liver Fibrosis, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Liver Fibrosis and special features on late-stage and discontinued projects. Drug profiles/records featured in the report undergoes periodic updation following a stringent set of processes that ensures that all the profiles are updated with the latest set of information. Additionally, processes including live news & deals tracking, browser based alert-box and clinical trials registries tracking ensure that the most recent developments are captured on a real time basis. Reasons to buy - Provides strategically significant competitor information, analysis, and insights to formulate effective R&D development strategies - Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage - Develop strategic initiatives by understanding the focus areas of leading companies - Identify and understand important and diverse types of therapeutics under development for Liver Fibrosis - Plan mergers and acquisitions effectively by identifying key players of the most promising pipeline - Devise corrective measures for pipeline projects by understanding Liver Fibrosis pipeline depth and focus of Indication therapeutics - Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline Companies Mentioned: - Genzyme - Gilead Sciences - Bioneer - FibroGen - Silence Therapeutics - Celgene - Nitto Denko - Genfit - Pharmaxis - La Jolla Pharmaceutical - ProMetic Life Sciences - LG Life Sciences - Digna Biotech - Advinus Therapeutics - Promedior - GenKyoTex - Angion Biomedica - Virobay - Intercept Pharmaceuticals - Tobira Therapeutics - GNI Group - Promethera Biosciences - BiOrion Technologies - ACROVIS biostructures - RXi Pharmaceuticals For more information visit http://www.researchandmarkets.com/research/wm...r_fibrosis
GILD: 101.90 (+1.10), ICPT: 209.12 (+7.12), TBRA: (), CELGZ: 3.17 (-0.04)
Ischemia Reperfusion Injury - Pipeline Review, H1 2014
M2 - Mon Dec 15, 3:56AM CST
Research and Markets (http://www.researchandmarkets.com/research/6pp4ft/ischemia) has announced the addition of the "Ischemia Reperfusion Injury - Pipeline Review, H1 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Ischemia Reperfusion Injury, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Ischemia Reperfusion Injury and special features on late-stage and discontinued projects. Drug profiles/records featured in the report undergoes periodic updation following a stringent set of processes that ensures that all the profiles are updated with the latest set of information. Additionally, processes including live news & deals tracking, browser based alert-box and clinical trials registries tracking ensure that the most recent developments are captured on a real time basis. Reasons to buy - Provides strategically significant competitor information, analysis, and insights to formulate effective R&D development strategies - Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage - Develop strategic initiatives by understanding the focus areas of leading companies - Identify and understand important and diverse types of therapeutics under development for Ischemia Reperfusion Injury - Plan mergers and acquisitions effectively by identifying key players of the most promising pipeline - Devise corrective measures for pipeline projects by understanding Ischemia Reperfusion Injury pipeline depth and focus of Indication therapeutics - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline Companies Mentioned: - Pharming Group N.V. - BioLineRx, Ltd. - Silence Therapeutics plc - Proteo, Inc. - Scynexis, Inc. - Prolong Pharmaceuticals - Trophos SA - Curatis Pharma GmbH - Alligator Bioscience AB - Opsona Therapeutics Ltd. - PledPharma AB - Zealand Pharma A/S - Omeros Corporation - Bolder Biotechnology, Inc. - Adienne S.r.l. - Genextra S.p.a. - KAEL-GemVax Co., Ltd. - Ischemix - APT Therapeutics, Inc. - Peptinnovate Limited For more information visit http://www.researchandmarkets.com/research/6pp4ft/ischemia
OMER: 20.63 (+0.13)
Hepatocellular Carcinoma - Pipeline Review, H2 2014
M2 - Mon Dec 01, 3:40AM CST
Research and Markets (http://www.researchandmarkets.com/research/8fjnjx/hepatocellular) has announced the addition of the "Hepatocellular Carcinoma - Pipeline Review, H2 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Hepatocellular Carcinoma, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Hepatocellular Carcinoma and special features on late-stage and discontinued projects. Reasons to buy - Provides strategically significant competitor information, analysis, and insights to formulate effective R&D development strategies - Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage - Develop strategic initiatives by understanding the focus areas of leading companies - Identify and understand important and diverse types of therapeutics under development for Hepatocellular Carcinoma - Plan mergers and acquisitions effectively by identifying key players of the most promising pipeline - Devise corrective measures for pipeline projects by understanding Hepatocellular Carcinoma pipeline depth and focus of Indication therapeutics - Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline Companies Mentioned: - 4SC - AbbVie - Acceleron Pharma - ACROVIS biostructures - Alfact Innovation - Alnylam - Amgen - AndroScienceration - ArQule - Arrowhead Researchration - Astex - AstraZeneca - Avidin Ltd - Bayer - Bio-Cancer Treatment - Blueprint Medicines - Boehringer Ingelheim - Boston Biomedical - Can-Fite BioPharma - CASI - CCRP Therapeutics - Celgeneration - Celsionration - China Medical System Holdings - Chroma Therapeutics - CrystalGenomics - CytRxration - Delcath Systems - Dicerna - Digna Biotech S.L. - Eisai. - Eli Lilly and Company - Endocyte - Exelixis - F. Hoffmann-La Roche - Gamida Cell - Geneluxration - Genoscience Pharma - GenSpera - GlaxoSmithKline - Green Cross Cellration - Immune Network - Immunicum AB - Immunomedics - Immunovative Therapies - Immuron - In Cell Art - Incuron LLC - Intezyne Inc - KAEL-GemVax. - KAHR medical - Kowa Company - La Jolla Pharmaceutical Company - Ligand - Lixte Biotechnology Holdings - MaxCyte - Merck KGaA - Millennium - MolMed S.p.A. - MultiCell Technologies - NeoStem - Nerviano Medical Sciences - Novartis - Nymox Pharmaceuticalration - Omerosration - Oncolys BioPharma - OncoTherapy Science - Ono Pharmaceutical. - Onxeo SA - Otsuka Holdings. - Panacea - Peregrine - Pfizer - PharmaEssentiaration - PhaseRx - Polaris - Priaxon - Progen - Provecs Medical - Provectus Biopharmaceuticals - Quantum - Raptor - Regulus Therapeutics - Rigontec - Santaris Pharma A/S - Sevion Therapeutics - Shanghai Sunway Biotech - Shenogen Pharma - Silence Therapeutics - SillaJen Co. - Simcere Pharmaceutical - Solasia Pharma K.K. - Synta - Taiwan Liposome Company - Tekmira - Therametrics holding - Therapure Biopharma - THERAVECTYS SA - Threshold - Toko Pharmaceutical Industries. - TRACON - Vaxon Biotech - Vertexrporated - Vicus Therapeutics LLC - Virttu Biologics For more information visit http://www.researchandmarkets.com/research/8f...tocellular
IMMU: 3.84 (unch), AMGN: 153.48 (+0.30), RGLS: 16.85 (-0.13), LLY: 70.56 (+0.03), GSK: 47.94 (+1.99), PVCT: 0.79 (-0.02), CANF: 3.85 (+0.05), DCTH: 1.14 (+0.04), NVS: 102.20 (-0.17), XLRN: 38.02 (+0.07)
Global RNA Based Therapeutics Market 2014-2020: Market to Grow at CAGR of 28% and Reach $1.2 Billion in 2020
M2 - Wed Oct 22, 5:44AM CDT
Research and Markets (http://www.researchandmarkets.com/research/4cwd5m/global_rna_based) has announced the addition of the "Global RNA Based Therapeutics Market (Technology, Application, End Users and Geography) - Size, Share, Global Trends, Company Profiles, Demand, Insights, Analysis, Research, Report, Opportunities, Segmentation and Forecast, 2013 - 2020" report to their offering. The RNA Based Therapeutics Market would reach $1.2 billion by 2020 registering a CAGR of 28.4% during 2014 - 2020 Due to its ability to render targeted solutions for chronic diseases such as cancer, AIDS, rare genetic disorders, and certain cardiovascular conditions, RNA based therapeutics is expected to significantly impact the global pharmaceutical industry. The large scale funding from public and private sector, growing interest of pharmaceuticals and biotech giants for developing novel delivery technology, and anticipated saving in healthcare expenditure are propelling the growth of RNA therapeutics market. Moreover, the platform technologies such as RNAi and antisense have enabled researchers to accelerate their research activities by defining gene sequences for chronic diseases. Companies have widened the research focus on RNA based drug as well, which has fueled the growth of the overall RNA therapeutics market.Pharmaceutical companies have identified the RNA therapeutics for variety of chronic diseases and are also exploring the potential of RNA technology for diagnostic purposes. The RNA based therapeutics market, in the near future is likely to witness a substantial level of momentum due to government funding and programs aimed at commercialization of these drugs. The government of United States, through its National Institute of Health (NIH), provides funds for RNA therapeutics research, thus assist in propelling the growth of this market. To boost the research activities the U.S. FDA is providing fast track designation to the RNA products, which are in the pipeline. The study suggests that the enabled technologies such as RNA interference technology (RNAi) and RNA antisense technology will dominate the market with RNAi technology getting interest from most of the participants. The development pipeline suggests that oncology segment will emerge as the largest application segment. This is largely due to the high prevalence of such diseases and limited efficiency of available therapeutics in treating such diseases. Scope of the Report MARKET BY TECHNOLOGY - Enabling technologies - Microarrays - Labeling - Purification - Linear amplification - qRT-PCR - Inhibition - Enabled technologies - RNA Interference (RNAi) technologies - Small interfering RNA (siRNA) - MicroRNA (miRNA) - RNA antisense technologies MARKET BY APPLICATION - Cardiovascular - Kidney Diseases - Oncology - Infectious diseases - Metabolic disorders - Others MARKET BY END USERS - Research - Therapeutics - Diagnosis Companies Mentioned - Alnylam Pharmaceuticals - Benitec Biopharma Limited (Australia) - Cenix BioScience GmbH (Germany) - Dicerna Pharmaceuticals - Genzyme Corporation (USA) (A Sanofi Company) - Quark Pharmaceuticals - Silence Therapeutics PLC (UK) - Sirnaomics - Tekmira Pharmaceuticals Corp. (Canada) For more information visit http://www.researchandmarkets.com/research/4c..._rna_based
DRNA: 21.30 (-0.06), TKMR: 21.45 (-0.22)
Metastatic Pancreatic Cancer - Pipeline Review, H2 2014
M2 - Wed Oct 08, 4:29AM CDT
Research and Markets (http://www.researchandmarkets.com/research/hnpdbv/metastatic) has announced the addition of the "Metastatic Pancreatic Cancer - Pipeline Review, H2 2014" report to their offering. This, Metastatic Pancreatic Cancer - Pipeline Review, H2 2014', provides an overview of the Metastatic Pancreatic Cancer's therapeutic pipeline. This report provides comprehensive information on the therapeutic development for Metastatic Pancreatic Cancer, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Metastatic Pancreatic Cancer and special features on late-stage and discontinued projects. This report features investigational drugs from across globe covering over 20 therapy areas and nearly 3,000 indications. The report is built using data and information sourced from This proprietary databases, Company/University websites, SEC filings, investor presentations and featured press releases from company/university sites and industry-specific third party sources, put together by This team. Drug profiles/records featured in the report undergoes periodic updation following a stringent set of processes that ensures that all the profiles are updated with the latest set of information. Additionally, processes including live news & deals tracking, browser based alert-box and clinical trials registries tracking ensure that the most recent developments are captured on a real time basis. Companies Mentioned: - F. Hoffmann-La Roche - AstraZeneca - Eli Lilly and Company - GlaxoSmithKline - Gilead Sciences - Merck & Co - FibroGen - Novartis AG - Pfizer Inc. - Silence Therapeutics plc - Celgene Corporation - Bayer AG - Incyte Corporation - Halozyme Therapeutics, Inc. - Momenta Pharmaceuticals, Inc. - Immunomedics, Inc. - Oncolytics Biotech Inc. - Synta Pharmaceuticals Corp. - NanoCarrier Co., Ltd. - Rexahn Pharmaceuticals, Inc. - Colby Pharmaceutical Company - INSYS Therapeutics, Inc. - OncoMed Pharmaceuticals, Inc. - Merrimack Pharmaceuticals, Inc. - Cornerstone Pharmaceuticals, Inc. - Pharma Mar, S.A. - Oncovir, Inc. - Cantex Pharmaceuticals, Inc. - Regulon Inc. - Fountain Biopharma Inc. - Oncozyme Pharma Inc. - AbbVie Inc. - Precision Biologics, Inc. For more information visit http://www.researchandmarkets.com/research/hnpdbv/metastatic
RNN: 0.75 (+0.01), IMMU: 3.84 (unch), ONCY: 0.51 (+0.02), OMED: 26.06 (+0.30), PFE: 34.64 (-0.23), INCY: 75.94 (+0.28), LLY: 70.56 (+0.03), GSK: 47.94 (+1.99), MNTA: 11.70 (-0.06), MACK: 10.22 (+0.40), SNTA: 2.29 (-0.03), INSY: 51.50 (-0.66), HALO: 14.73 (+0.23), GILD: 101.90 (+1.10), MRK: 58.81 (-0.07), ABBV: 58.05 (+1.00), NVS: 102.20 (-0.17), CELGZ: 3.17 (-0.04)
Amarantus elects Iain Ross to board
M2 - Mon Sep 01, 3:29AM CDT
Biotechnology company Amarantus Bioscience Holdings (Other OTC:AMBS) revealed on Friday the addition of Iain Ross to its board of directors.
Pulmonary Hypertension - Pipeline Review, H1 2014
M2 - Wed Jun 25, 4:09AM CDT
Research and Markets (http://www.researchandmarkets.com/research/289bcz/pulmonary) has announced the addition of the "Pulmonary Hypertension - Pipeline Review, H1 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Pulmonary Hypertension, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Pulmonary Hypertension and special features on late-stage and discontinued projects. Drug profiles/records featured in the report undergoes periodic updation following a stringent set of processes that ensures that all the profiles are updated with the latest set of information. Additionally, processes including live news & deals tracking, browser based alert-box and clinical trials registries tracking ensure that the most recent developments are captured on a real time basis. Reasons to buy - Provides strategically significant competitor information, analysis, and insights to formulate effective R&D development strategies - Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage - Develop strategic initiatives by understanding the focus areas of leading companies - Identify and understand important and diverse types of therapeutics under development for Pulmonary Hypertension - Plan mergers and acquisitions effectively by identifying key players of the most promising pipeline - Devise corrective measures for pipeline projects by understanding Pulmonary Hypertension pipeline depth and focus of Indication therapeutics - Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline Companies Mentioned: - Activaero GmbH - Bayer AG - Ikaria Inc. - Proreo Pharma AG - Sanofi - Silence Therapeutics plc - Vicore Pharma AB For more information visit http://www.researchandmarkets.com/research/289bcz/pulmonary



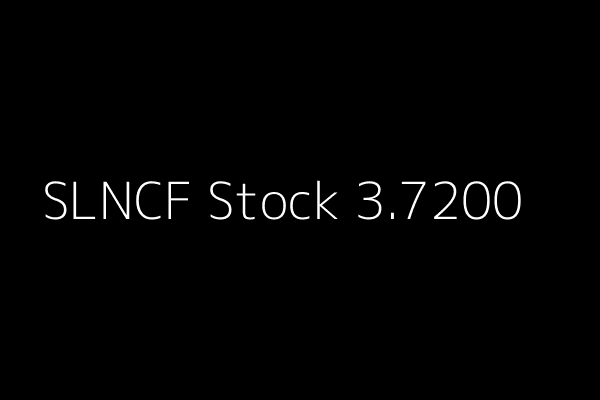
 (0)
(0) (0)
(0)