RGRX News Alert Regenerx Biopharmaceuticals Inc (RGRX) 0.2000
Post# of 64074
Sensory Neuropathy Therapeutics Pipeline Market H1 2015 Review Report Available at RnRMarketResearch.com
PRWeb - Tue Feb 10, 7:31AM CST
The report "Peripheral Neuropathy (Sensory Neuropathy) - Pipeline Review, H1 2015″ provides comprehensive information on the therapeutic development for Peripheral Neuropathy (Sensory Neuropathy). Peripheral Neuropathy (Sensory Neuropathy) is a damage to one or more of peripheral nerves that includes motor, sensory and autonomic fibers. The cause of peripheral neuropathy is often unidentified. The most widespread causes of peripheral neuropathy are diabetes, shingles, kidney failure, various drugs and vitamin shortage. When the peripheral sensory nerves are damaged, they fail to send sensory messages (pain, heat, touch, cold etc). Neuropathy occurs in 60% to 70% of people with diabetes. The report strengthens R&D pipelines by identifying new targets and MOAs to produce first-in-class and best-in-class products. Complete report is available @ http://www.rnrmarketresearch.com/peripheral-n...eport.html .
PFE: 34.64 (-0.23)
RegeneRx & G-treeBNT partnership to develop RGN-259 in the US for ophthalmic indications
M2 - Fri Jan 30, 2:14AM CST
Biopharmaceutical company RegeneRx Biopharmaceuticals (Other OTC:RGRX) reported on Wednesday the launch of an agreement to develop RGN-259 for dry eye syndrome and neurotrophic keratitis (an orphan indication) in the US in partnership with Korean-based pharma company G-treeBNT Co.
RegeneRx and G-treeBNT Create Joint Venture to Develop RGN-259 in the U.S.
PR Newswire - Wed Jan 28, 11:11AM CST
RegeneRx Biopharmaceuticals, Inc. (OTCQB: RGRX) ("the Company" or "RegeneRx"
RegeneRx Partner Submits Phase IIB/III IND to Korean Health Authority for RGN-259 for the Treatment of Dry Eye Syndrome
PR Newswire - Mon Dec 29, 7:30AM CST
RegeneRx Biopharmaceuticals, Inc. (OTCQB: RGRX) ("RegeneRx"
Researchers Report Thymosin Beta 4 is Cardioprotective in Pulmonary Hypertension/Heart Failure Model
PR Newswire - Tue Nov 25, 7:43AM CST
RegeneRx Biopharmaceuticals, Inc. (OTCQB: RGRX) ("the Company" or "RegeneRx"
RegeneRx Announces Management Changes
PR Newswire - Thu Nov 06, 3:05PM CST
RegeneRx Biopharmaceuticals, Inc. (OTCQB: RGRX) ("the Company" or "RegeneRx"
U.S. Patent Office Significantly Extends the Term of Three RegeneRx Patents
PR Newswire - Mon Nov 03, 8:00AM CST
RegeneRx Biopharmaceuticals, Inc. (OTCQB: RGRX)("the Company" or "RegeneRx"
Dr. Michael Chopp Reports on Successful Remodeling of the Central and Peripheral Nervous Systems after Injury/Disease Using Thymosin Beta 4
PR Newswire - Thu Oct 23, 12:00PM CDT
RegeneRx Biopharmaceuticals, Inc. (OTCBB: RGRX) ("the Company" or "RegeneRx"
RegeneRx to begin Phase 3 study of RGN-259 eye drops in neurotrophic keratopathy patients
M2 - Tue Oct 21, 7:29AM CDT
Therapeutic peptide company RegeneRx Biopharmaceuticals (Other OTC:RGRX) said on Monday that it plans to start its Phase 3 by mid-2015 to evaluate RGN-259 preservative-free eye drops for the treatment of patients with neurotrophic keratopathy (NK), an orphan disease of the cornea, following its September meeting with the US FDA.
RegeneRx Allowed to Proceed with Phase 3 Using RGN-259 Eye Drops for the Treatment of Neurotrophic Keratopathy
PR Newswire - Mon Oct 20, 9:12AM CDT
RegeneRx Biopharmaceuticals, Inc. (OTC: RGRX) ("the Company" or "RegeneRx"
RegeneRx awarded patent for Inhibition or Reversal of Skin Aging in Canada
M2 - Wed Oct 01, 6:07AM CDT
Therapeutic peptide company RegeneRx Biopharmaceuticals (Other OTC :RGRX) revealed on Tuesday the receipt of the Canadian Patent for Inhibition or Reversal of Skin Aging by Actin-Sequestering Peptides.
RegeneRx Receives Canadian Patent for Inhibition or Reversal of Skin Aging
PR Newswire - Tue Sep 30, 9:00AM CDT
RegeneRx Biopharmaceuticals, Inc. (OTC: RGRX) ("the Company" or "RegeneRx"
Global Venous Leg Ulcers (Crural ulcer) Therapeutics Pipeline Review 2014 - 11 Companies & 12 Drug Profiles
M2 - Thu Sep 11, 4:32AM CDT
Research and Markets (http://www.researchandmarkets.com/research/hdzqtm/venous_leg_ulcers) has announced the addition of the "Venous Leg Ulcers (Crural ulcer) - Pipeline Review, H2 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Venous Leg Ulcers (Crural ulcer), complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Venous Leg Ulcers (Crural ulcer) and special features on late-stage and discontinued projects. The report enhances decision making capabilities and help to create effective counter strategies to gain competitive advantage. It strengthens R&D pipelines by identifying new targets and MOAs to produce first-in-class and best-in-class products. Companies Involved in Therapeutics Development - Smith & Nephew Plc - CardioVascular BioTherapeutics, Inc. - Stratatech Corporation - RegeneRx Biopharmaceuticals, Inc. - CytoTools AG - FirstString Research, Inc. - CoDa Therapeutics, Inc. - Adocia - Intralytix, Inc. - MacroCure - Pergamum AB Drug Profiles - HP-802247 - CureXcell - Granexin - CVBT-141B - RGN-137 - DermaPro CL-05 - CODA-001 - LL-37 - Stem Cell Therapy for Venous Leg Ulcers - WPP-201 - Biochaperone PDGF-BB - Cathelicidin For more information visit http://www.researchandmarkets.com/research/hd...leg_ulcers
SNN: 35.82 (-0.28)
RegeneRx Receives $1 Million Pursuant to Product License and Stock Purchase Agreement
PR Newswire - Wed Sep 03, 7:45AM CDT
RegeneRx Biopharmaceuticals, Inc. (OTC: RGRX) ("the Company" or "RegeneRx"
Epidermolysis Bullosa - Pipeline Review, H1 2014
M2 - Wed Jul 30, 3:10AM CDT
Research and Markets (http://www.researchandmarkets.com/research/xjhcc9/epidermolysis) has announced the addition of the "Epidermolysis Bullosa - Pipeline Review, H1 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Epidermolysis Bullosa, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Epidermolysis Bullosa and special features on late-stage and discontinued projects. Drug profiles/records featured in the report undergoes periodic updation following a stringent set of processes that ensures that all the profiles are updated with the latest set of information. Additionally, processes including live news & deals tracking, browser based alert-box and clinical trials registries tracking ensure that the most recent developments are captured on a real time basis. Reasons to buy - Provides strategically significant competitor information, analysis, and insights to formulate effective R&D development strategies - Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage - Develop strategic initiatives by understanding the focus areas of leading companies - Identify and understand important and diverse types of therapeutics under development for Epidermolysis Bullosa - Plan mergers and acquisitions effectively by identifying key players of the most promising pipeline - Devise corrective measures for pipeline projects by understanding Epidermolysis Bullosa pipeline depth and focus of Indication therapeutics - Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline Companies Mentioned: - RegeneRx Biopharmaceuticals, Inc. - Fibrocell Science, Inc. - Birken AG - Scioderm, Inc. For more information visit http://www.researchandmarkets.com/research/xj...dermolysis
FCSC: 4.75 (+0.50)
New Report: Thymosin B4 Prevents Heart Rupture & Improves Cardiac Function after Heart Attack in Mice
Business Wire - Tue Jul 15, 8:02AM CDT
RegeneRx Biopharmaceuticals, Inc. (OTC: RGRX) ("the Company" or "RegeneRx"
RegeneRx's Strategic Partner G-treeBNT Preparing for Phase III Dry Eye Trials in Asia with RGN-259
Business Wire - Mon Jul 07, 7:30AM CDT
RegeneRx Pharmaceuticals (OTCBB:RGRX), and G-treeBNT Co. Ltd., a Korean biotech firm, jointly announced today that G-treeBNT is preparing to file an IND and sponsor a Phase III clinical trial with RGN-259 (a Thymosin Beta 4-based preservative-free eye drop) in patients with moderate to severe dry eye initially in South Korea, followed by Japan and Australia, with follow-on registrations in certain additional Asian and Pacific Rim countries if appropriate.
RegeneRx and Lee's Pharmaceutical Dry Eye Drug Progresses - Analyst Blog
Zacks Equity Research - Zacks Investment Research - Mon Jun 23, 11:10AM CDT
RegeneRx Biopharmaceuticals, Inc. (RGRX) and Lee's Pharmaceutical announced that the Chinese regulatory authority has accepted a phase II investigational new drug application for RGN-259.
GILD: 101.90 (+1.10), ALXN: 182.29 (+6.02), REGN: 402.40 (+0.66)
RegeneRx Biopharmaceuticals to begin Phase 2 trial of RGN-259 in dry eye syndrome patients in China in Q1 2015
M2 - Mon Jun 23, 5:25AM CDT
Novel therapeutic company RegeneRx Biopharmaceuticals (Other OTC:RGRX) and Lee's Pharmaceutical Holdings jointly announced on Friday that they will begin the Phase II IND for RGN-259 in patients with moderate to severe dry eye syndrome in China.
Chinese FDA Accepts Phase 2 IND for RGN-259 for Dry Eye
Business Wire - Sun Jun 22, 11:00PM CDT
RegeneRx Biopharmaceuticals, Inc. (OTC: RGRX) ("RegeneRx"



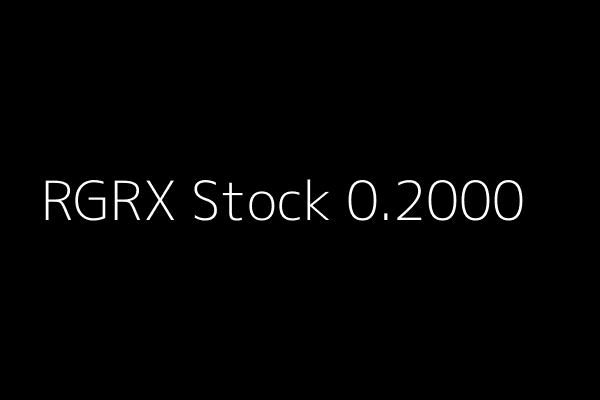
 (0)
(0) (0)
(0)