QRXPF News Alert Qrxpharma Ltd (QRXPF) 0.0080 02/15/2015 10:
Post# of 64200
Hemorrhage - Pipeline Review, H2 2013
M2 - Mon Dec 15, 3:34AM CST
Research and Markets (http://www.researchandmarkets.com/research/fjlpxn/hemorrhage) has announced the addition of the "Hemorrhage - Pipeline Review, H2 2013" report to their offering. 'Hemorrhage - Pipeline Review, H2 2013', provides an overview of the indication's therapeutic pipeline. This report provides information on the therapeutic development for Hemorrhage, complete with latest updates, and special features on late-stage and discontinued projects. It also reviews key players involved in the therapeutic development for Hemorrhage. Hemorrhage - Pipeline Review, Half Year is built using data and information sourced from proprietary databases, Company/University websites, SEC filings, investor presentations and featured press releases from company/university sites and industry-specific third party sources, put together by our team. Scope - A snapshot of the global therapeutic scenario for Hemorrhage. - A review of the Hemorrhage products under development by companies and universities/research institutes based on information derived from company and industry-specific sources. - Coverage of products based on various stages of development ranging from discovery till registration stages. - A feature on pipeline projects on the basis of monotherapy and combined therapeutics. - Coverage of the Hemorrhage pipeline on the basis of route of administration and molecule type. - Key discontinued pipeline projects. - Latest news and deals relating to the products. Reasons to buy - Identify and understand important and diverse types of therapeutics under development for Hemorrhage. - Identify emerging players with potentially strong product portfolio and design effective counter-strategies to gain competitive advantage. - Plan mergers and acquisitions effectively by identifying players of the most promising pipeline. - Devise corrective measures for pipeline projects by understanding Hemorrhage pipeline depth and focus of Indication therapeutics. - Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope. - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline. Companies Mentioned: - Grifols, S.A. - Digna Biotech, S.L. - Thrombotargets Corporation - QRxPharma Limited For more information visit http://www.researchandmarkets.com/research/fjlpxn/hemorrhage
Parkinson's Disease - Pipeline Review, H2 2014
M2 - Tue Dec 02, 5:55AM CST
Research and Markets (http://www.researchandmarkets.com/research/7k9xvt/parkinsons) has announced the addition of the "Parkinson's Disease - Pipeline Review, H2 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Parkinson's Disease, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. Reasons to buy - Provides strategically significant competitor information, analysis, and insights to formulate effective R&D development strategies - Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage - Develop strategic initiatives by understanding the focus areas of leading companies - Identify and understand important and diverse types of therapeutics under development for Parkinson's Disease - Plan mergers and acquisitions effectively by identifying key players of the most promising pipeline - Devise corrective measures for pipeline projects by understanding Parkinson's Disease pipeline depth and focus of Indication therapeutics - Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope - Modify the therapeutic portfolio by identifying discontinued projects and understanding the factors that drove them from pipeline Companies Mentioned: 169 companies are cited in this text including: - Amicus Therapeutics, Inc. - AstraZeneca PLC - Bial - Portela & Ca, S.A. - Calico LLC - Cardax Pharmaceuticals, Inc. - Edison Pharmaceuticals, Inc. - GlaxoSmithKline plc - GliaCure Inc. - H. Lundbeck A/S - Io Therapeutics, Inc. - Iproteos S.L. - Jeil Pharmaceutical Co., Ltd. - KineMed, Inc. - Medeia Therapeutics Ltd. - Motac Neuroscience Ltd - Netherlands Translational Research Center B.V. - nLife Therapeutics, S.L. - Novartis AG - OPKO Health, Inc. - Orion Oyj - Peptron, Inc. - Pfizer Inc. - QR Pharma, Inc. - QRxPharma Limited - Raptor Pharmaceuticals Corp. - SanBio, Inc. - Saneron CCEL Therapeutics, Inc. - Stelic Institute & Co. - Sumitomo Dainippon Pharma Co., Ltd. For more information visit http://www.researchandmarkets.com/research/7k9xvt/parkinsons
RPTP: 9.05 (-0.40), KNMD: (), OPK: 13.80 (-0.28), PFE: 34.64 (-0.23), AZN: 69.96 (+0.20), GSK: 47.94 (+1.99), FOLD: 8.36 (-0.11), NVS: 102.20 (-0.17)
QRxPharma Provides Feedback On End-of-Review Meeting With The FDA On Moxduo®
PR Newswire - Fri Jul 11, 8:00AM CDT
QRxPharma Limited (ASX: QRX and OTCQX: QRXPY) today provided feedback on its 9 July End-of-Review (EOR) meeting with the United States Food and Drug Administration (FDA). The meeting was held to discuss the feasibility and requirements for approving Moxduo, an immediate release Dual Opioid®, for the treatment of moderate to severe acute pain.
QRxPharma Announces Board Resignations
PR Newswire - Wed Jul 09, 8:02AM CDT
QRxPharma Limited (ASX: QRX and OTCQX: QRXPY) announced today that Chairman, Dr. Peter Farrell and Director, Dr. Gary Pace have resigned from the QRxPharma board effective at 9:50am (AEST) 9 July 2014 prior to commencement of the General Meeting (GM) requisitioned by shareholders associated with Langley Walker. It is expected that Bruce Hancox and Dr. Richard Treagus will be appointed as directors pursuant to resolutions in the requisitioned notice of meeting, with those appointments taking effect from the close of the meeting.
Dystonia - Pipeline Review, H1 2014
M2 - Thu Jun 26, 3:21AM CDT
Research and Markets (http://www.researchandmarkets.com/research/mgqh87/dystonia) has announced the addition of the "Dystonia - Pipeline Review, H1 2014" report to their offering. This report provides comprehensive information on the therapeutic development for Dystonia, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Dystonia and special features on late-stage and discontinued projects. Drug profiles/records featured in the report undergoes periodic updation following a stringent set of processes that ensures that all the profiles are updated with the latest set of information. Additionally, processes including live news & deals tracking, browser based alert-box and clinical trials registries tracking ensure that the most recent developments are captured on a real time basis. The report enhances decision making capabilities and help to create effective counter strategies to gain competitive advantage. It strengthens R&D pipelines by identifying new targets and MOAs to produce first-in-class and best-in-class products. Scope - The report provides a snapshot of the global therapeutic landscape of Dystonia - The report reviews key pipeline products under drug profile section which includes, product description, MoA and R&D brief, licensing and collaboration details & other developmental activities - The report reviews key players involved in the therapeutics development for Dystonia and enlists all their major and minor projects - The report summarizes all the dormant and discontinued pipeline projects - A review of the Dystonia products under development by companies and universities/research institutes based on information derived from company and industry-specific sources - Pipeline products coverage based on various stages of development ranging from pre-registration till discovery and undisclosed stages Companies Mentioned: - Addex Therapeutics - Advicenne Pharma SA - EpiVax, Inc. - Ipsen S.A. - QRxPharma Limited For more information visit http://www.researchandmarkets.com/research/mgqh87/dystonia



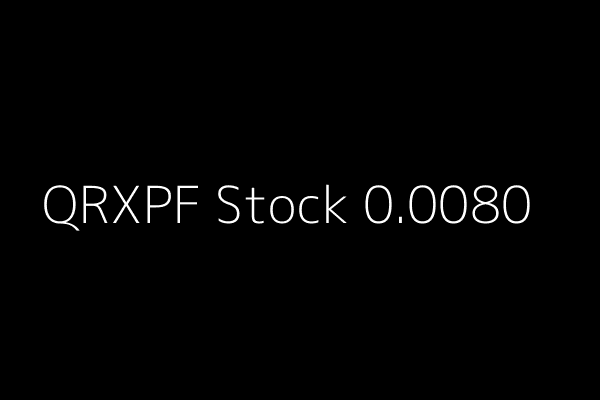
 (0)
(0) (0)
(0)