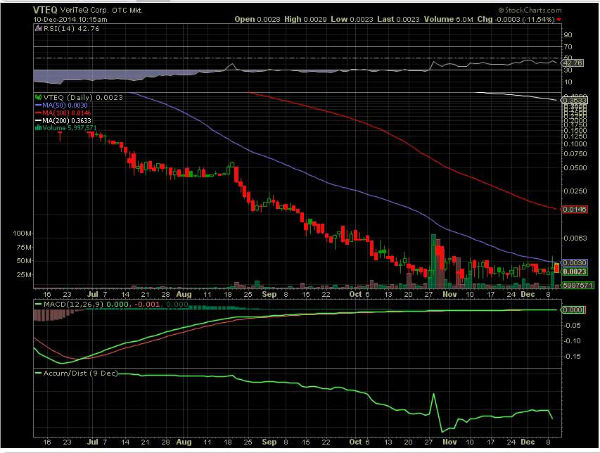
VTEQ ~ "FDA cleared" Q Inside Safety Technology!
Post# of 89713
Q Inside Safety Technology Growth Accelerates as Over 5,000 Women Have Now Received Motiva Breast Implants With VeriTeQ's Technology
Procedures Increase Approximately 400% Since September 2014
VeriTeQ Corporation
December 9, 2014 9:00 AM
GlobeNewswire
DELRAY BEACH, Fla., Dec. 9, 2014 (GLOBE NEWSWIRE) -- VeriTeQ Corporation (OTC Markets:VTEQ) ("VeriTeQ"
VeriTeQ's FDA cleared Q Inside Safety Technology(TM) acts as an electronic serial number in breast implants and other implantable and reusable medical devices to provide physicians and patients access to secure online databases to retrieve device-specific data, such as serial number, manufacturer name, date of manufacture, lot number, volume, size, and other data from the medical device manufacturer. Q Inside Safety Technology(TM) may also provide an extra level of protection to the patient in the event of a recall or other safety event.
http://finance.yahoo.com/news/q-inside-safety...00969.html
 (0)
(0) (0)
(0)