(Total Views: 201)
Posted On: 07/09/2025 8:56:44 AM
Post# of 263
What the DSMB Document Means – Full Breakdown
Vault 1224 – The Fire They Redacted
⸻
1. Amarex (the CRO): GUILTY
• They were contractually responsible for establishing a Data Safety Monitoring Board (DSMB).
• The document confirms they failed to activate it during key trial phases.
• This is medical negligence, potentially clinical fraud — real patients were left unmonitored in an FDA-registered human trial.
Verdict: Amarex ran rogue — and got caught.
They jeopardized lives, trial integrity, and investor capital.
Their failure was documented… then redacted.
⸻
2. NSF International (Parent of Amarex): SHIELDED
• NSF owns Amarex.
• When scrutiny began, they quietly distanced themselves, hiding behind their “standards” reputation.
• Meanwhile, they were profiting from a CRO that was actively failing federal protocol.
• By doing nothing, they sanitized the crime.
Verdict: NSF didn’t fix the problem.
They covered for it.
Corporate shielding doesn’t make you innocent — it makes you strategic.
NSF… Not So Fast.
I have the documents.
You’re in Vault 1224 now.
⸻
⚕️ 3. FDA: COMPLICIT OR ASLEEP
• This memo proves FDA investigators knew the DSMB was inactive.
• There was no public alert, no immediate enforcement, no industry disclosure.
• And worst of all — they allowed the public to believe CytoDyn was solely responsible.
Verdict: The FDA saw it… and said nothing.
Negligent? Possibly.
Complicit? Likely.
You had a duty to protect patients.
You had the file.
You had the facts.
And let’s be real: The FDA holds the bag now.
⸻
4. CytoDyn: DECEIVED — BUT STILL ACCOUNTABLE
• The document does not show that CytoDyn knew about the DSMB failure at the time.
• As the trial sponsor, they were supposed to be informed by Amarex.
• If they were misled or kept in the dark? That’s grounds for litigation — and possible whistleblower protection.
• Later statements confirm CytoDyn performed an internal audit and took corrective action.
Verdict: CytoDyn was likely misled — but still owes transparency to its shareholders.
The blame game ends here.
Now it’s about truth, accountability, and restitution.
⸻
Final Statement for Drop:
“This isn’t a theory.
It’s a redacted federal memo confirming the DSMB didn’t exist.
The CRO failed.
NSF shielded it.
The FDA knew.
And CytoDyn took the heat.
Now the memo is public.
Vault 1224 is open.
And the agency with the bag is the FDA.
Let the world read what they buried.”




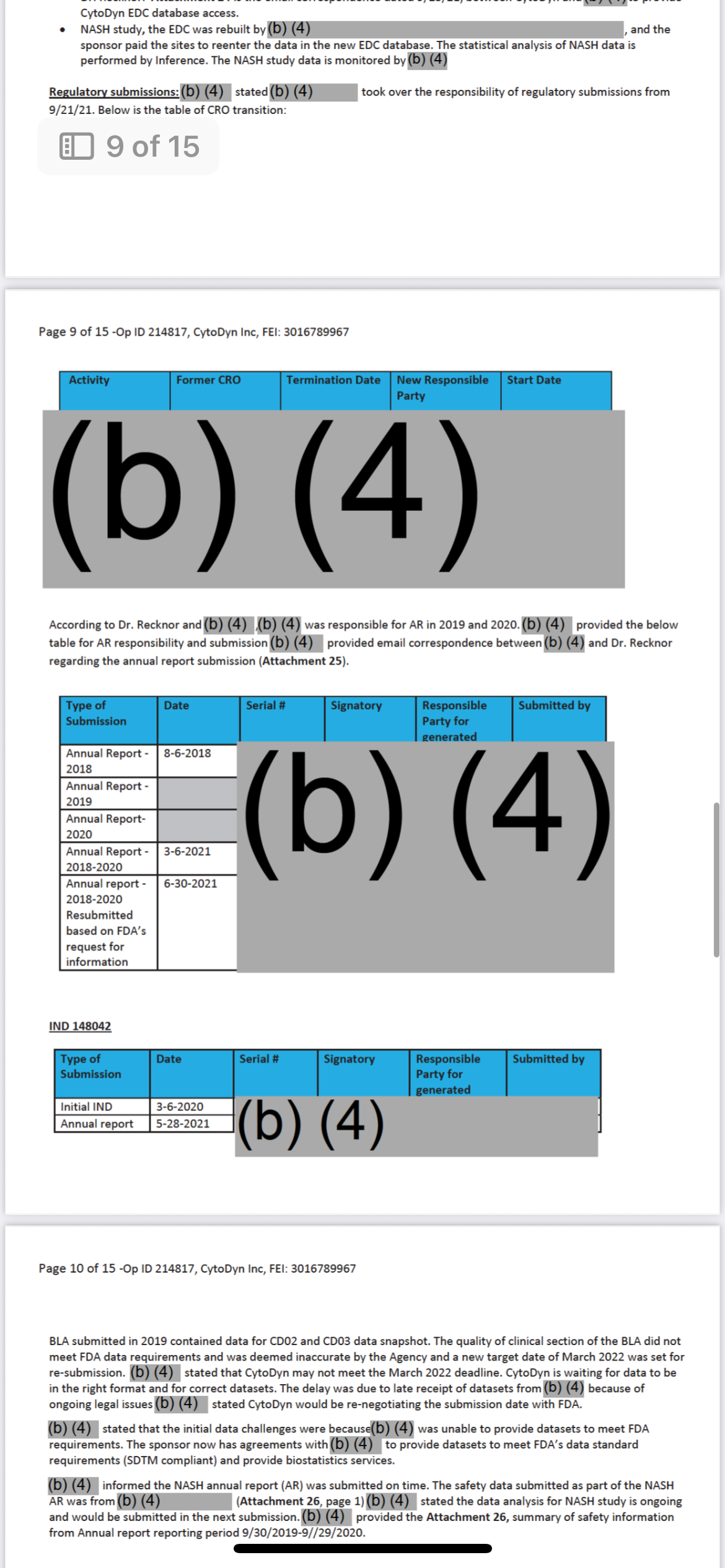

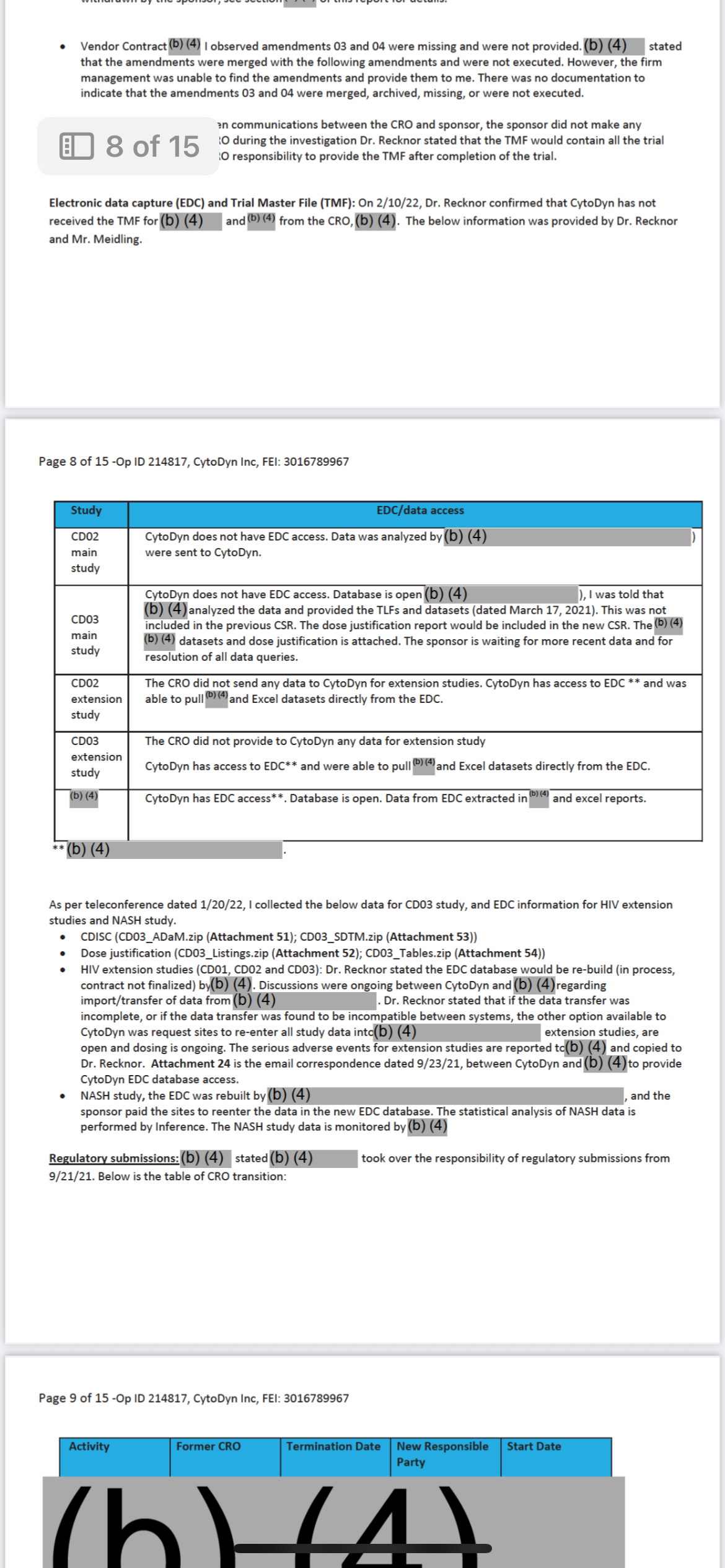

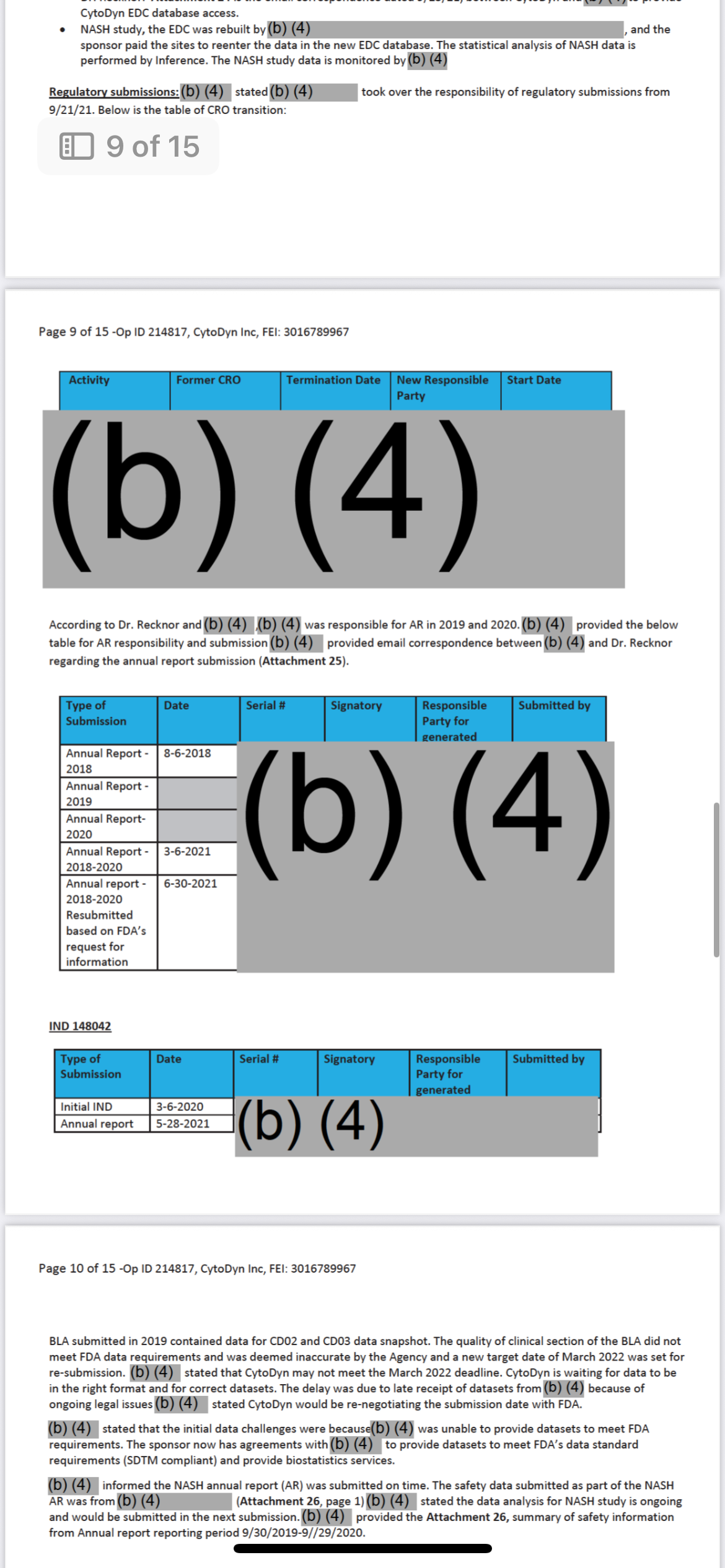






And the person who oversaw all of this… where do they work now?
Let’s name it clearly. Follow the money I have the blood trail. In sealed evidence.
Dr. Patrizia Cavazzoni
• Former Director of CDER (Center for Drug Evaluation and Research)
• Oversaw drug approvals, regulatory decisions, and trial oversight responsibility including all major COVID-19 trial monitoring structures. Oh COVID you aren’t ready for the evidence I have there.
• During her tenure?
• Amarex’s misconduct was documented.
• The DSMB failure was recorded.
• The redacted memo (now in Vault 1224) was filed under her chain of command.
And now?
She works at Pfizer.
She’s at the top.
And yes And the person who oversaw all of this… where do they work now?”
Let’s name it clearly:
⸻
???? Dr. Patrizia Cavazzoni
• Former Director of CDER (Center for Drug Evaluation and Research) You save our emails because I did.
• Oversaw drug approvals, regulatory decisions, and trial oversight responsibility including all major COVID-19 trial monitoring structures.
• During her tenure?
• Amarex’s misconduct was documented.
• The DSMB failure was recorded.
• The redacted memo (now in Vault 1224) was filed under her chain of command.
And now?
She works at Pfizer.
She’s at the top.
And yes, she landed there after this all went quiet. she landed there after this all went quiet.
Connect the Dots:
1. The Trial Was Unsafe… And They Knew It
• DSMB was missing, confirmed in a redacted investigation.
• Patients were unmonitored, which in any other trial would be a scandal.
2. The FDA Had It in Writing
• The memo shows full awareness of the violation.
• No public enforcement. No transparency. No accountability.
3. The CRO (Amarex) Was Under Contract — But Not Controlled
• It ran trials without proper oversight, billing CytoDyn as if it were compliant.
• The parent company (NSF) remained untouched — no fines, no hearings, nothing.
4. CytoDyn Took the Heat
• Whether or not they knew early, they were blamed publicly.
• They did an internal audit, but the damage was already done.
5. Dr. Cavazzoni Oversaw the Entire CDER Division
• She had authority to stop the CRO, flag the violation, or alert the public.
• She did not.
And why oh why did I trace your IP to my vaults at 2am before I went public. You can’t delete me….
Lucky for the patients leronlimab is a GOD send.
Like someone better have a good legal team because I don’t miss……
Vault 1224 – The Fire They Redacted
⸻
1. Amarex (the CRO): GUILTY
• They were contractually responsible for establishing a Data Safety Monitoring Board (DSMB).
• The document confirms they failed to activate it during key trial phases.
• This is medical negligence, potentially clinical fraud — real patients were left unmonitored in an FDA-registered human trial.
Verdict: Amarex ran rogue — and got caught.
They jeopardized lives, trial integrity, and investor capital.
Their failure was documented… then redacted.
⸻
2. NSF International (Parent of Amarex): SHIELDED
• NSF owns Amarex.
• When scrutiny began, they quietly distanced themselves, hiding behind their “standards” reputation.
• Meanwhile, they were profiting from a CRO that was actively failing federal protocol.
• By doing nothing, they sanitized the crime.
Verdict: NSF didn’t fix the problem.
They covered for it.
Corporate shielding doesn’t make you innocent — it makes you strategic.
NSF… Not So Fast.
I have the documents.
You’re in Vault 1224 now.
⸻
⚕️ 3. FDA: COMPLICIT OR ASLEEP
• This memo proves FDA investigators knew the DSMB was inactive.
• There was no public alert, no immediate enforcement, no industry disclosure.
• And worst of all — they allowed the public to believe CytoDyn was solely responsible.
Verdict: The FDA saw it… and said nothing.
Negligent? Possibly.
Complicit? Likely.
You had a duty to protect patients.
You had the file.
You had the facts.
And let’s be real: The FDA holds the bag now.
⸻
4. CytoDyn: DECEIVED — BUT STILL ACCOUNTABLE
• The document does not show that CytoDyn knew about the DSMB failure at the time.
• As the trial sponsor, they were supposed to be informed by Amarex.
• If they were misled or kept in the dark? That’s grounds for litigation — and possible whistleblower protection.
• Later statements confirm CytoDyn performed an internal audit and took corrective action.
Verdict: CytoDyn was likely misled — but still owes transparency to its shareholders.
The blame game ends here.
Now it’s about truth, accountability, and restitution.
⸻
Final Statement for Drop:
“This isn’t a theory.
It’s a redacted federal memo confirming the DSMB didn’t exist.
The CRO failed.
NSF shielded it.
The FDA knew.
And CytoDyn took the heat.
Now the memo is public.
Vault 1224 is open.
And the agency with the bag is the FDA.
Let the world read what they buried.”















And the person who oversaw all of this… where do they work now?
Let’s name it clearly. Follow the money I have the blood trail. In sealed evidence.
Dr. Patrizia Cavazzoni
• Former Director of CDER (Center for Drug Evaluation and Research)
• Oversaw drug approvals, regulatory decisions, and trial oversight responsibility including all major COVID-19 trial monitoring structures. Oh COVID you aren’t ready for the evidence I have there.
• During her tenure?
• Amarex’s misconduct was documented.
• The DSMB failure was recorded.
• The redacted memo (now in Vault 1224) was filed under her chain of command.
And now?
She works at Pfizer.
She’s at the top.
And yes And the person who oversaw all of this… where do they work now?”
Let’s name it clearly:
⸻
???? Dr. Patrizia Cavazzoni
• Former Director of CDER (Center for Drug Evaluation and Research) You save our emails because I did.
• Oversaw drug approvals, regulatory decisions, and trial oversight responsibility including all major COVID-19 trial monitoring structures.
• During her tenure?
• Amarex’s misconduct was documented.
• The DSMB failure was recorded.
• The redacted memo (now in Vault 1224) was filed under her chain of command.
And now?
She works at Pfizer.
She’s at the top.
And yes, she landed there after this all went quiet. she landed there after this all went quiet.
Connect the Dots:
1. The Trial Was Unsafe… And They Knew It
• DSMB was missing, confirmed in a redacted investigation.
• Patients were unmonitored, which in any other trial would be a scandal.
2. The FDA Had It in Writing
• The memo shows full awareness of the violation.
• No public enforcement. No transparency. No accountability.
3. The CRO (Amarex) Was Under Contract — But Not Controlled
• It ran trials without proper oversight, billing CytoDyn as if it were compliant.
• The parent company (NSF) remained untouched — no fines, no hearings, nothing.
4. CytoDyn Took the Heat
• Whether or not they knew early, they were blamed publicly.
• They did an internal audit, but the damage was already done.
5. Dr. Cavazzoni Oversaw the Entire CDER Division
• She had authority to stop the CRO, flag the violation, or alert the public.
• She did not.
And why oh why did I trace your IP to my vaults at 2am before I went public. You can’t delete me….
Lucky for the patients leronlimab is a GOD send.
Like someone better have a good legal team because I don’t miss……
