(Total Views: 178)
Posted On: 03/26/2023 11:57:49 AM
Post# of 394
The Periodic Table .............................................
https://investorshangout.com/images/MYImages/...cTable.png
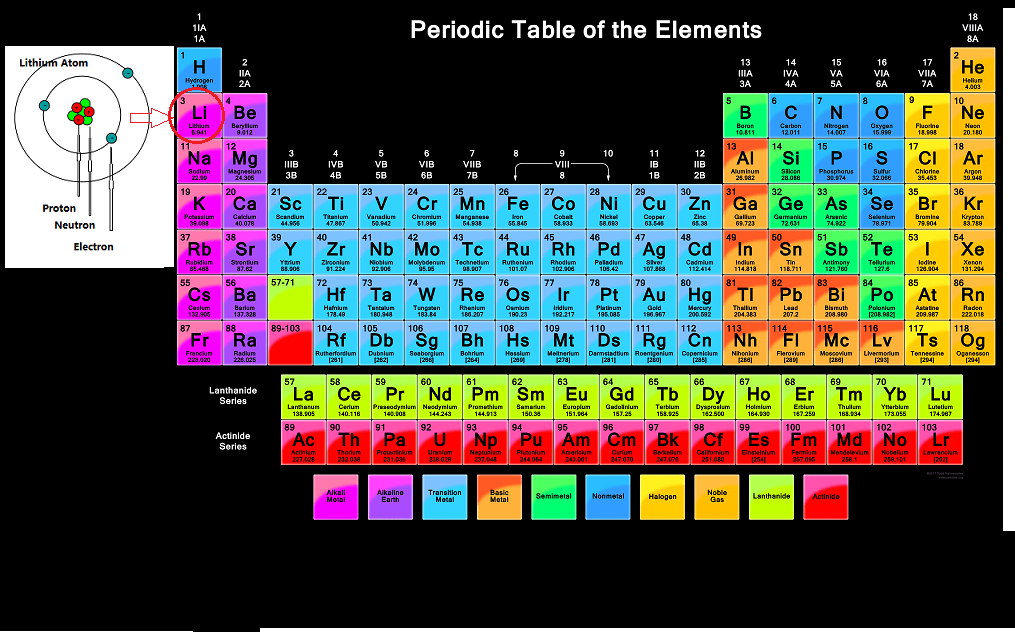
Chemistry of Lithium
Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 grams/mole. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - 3 = ~4). One mole contains 6.022 x 10 to the power 23 atoms.
https://chem.libretexts.org/Bookshelves/Inorg...203%20%3D%

https://investorshangout.com/images/MYImages/...cTable.png
Chemistry of Lithium
Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 grams/mole. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - 3 = ~4). One mole contains 6.022 x 10 to the power 23 atoms.
https://chem.libretexts.org/Bookshelves/Inorg...203%20%3D%
