(Total Views: 819)
Posted On: 02/28/2022 2:32:57 PM
Post# of 157566
Re: USS JOHNSTON #119286
Great question, and it's too bad they haven't been clear on all the numbers.
The 8/25/21 PR says:
[Note it says "modified" Overall Survival, but they probably mean median overall survival.]
Thus, the 980% increase in mOS seems to be an upper bound ("as much as" or "up to" ...) of a confidence interval, using unknown numbers and unknown statistic (does not seem to be simply months of survival, rather some kind of hazard ratio), and apparently using 23 of the 30 combined subjects with low/decreased CTCs.
The 11/3/21 PR one-upped the "980% increase" to "3600%!"
https://www.cytodyn.com/newsroom/press-releas...ysis-of-28
This new analysis removed 2 of the 30 from the analysis to get 28. I forget why they did this.
The 11/8/21 PR gives a hint of where this 980% and 3600% comes from:
https://www.cytodyn.com/newsroom/press-releas...esignation
The 36.0 hazard ratio I believe is where he gets the 3600% from (and probably the 980% too). 3600% is the hazard ratio for mOS for those 21 of the 28 combined subjects with low CTCs at baseline (3) or after LL treatment (18), compared to some unspecified historical cohort (or is it compared to the 7 without low CTCs?). 13 of these 28 were from the mTNBC trial (combo with carboplatin), and the rest from compassionate use or the basket trial, at different stages of mTNBC disease.
The confidence intervals given for mOS are apparently on the HR, not months of median overall survival. The hazard ratio gives the ratio of probability of death for those with low CTCs compared to high CTCs (N=21/28 apparently compared to N=7/28, or perhaps compared to some unspecified historical cohort?) using Cox proportional hazards model and Kaplan Meier survival curves, as done below.
I think they are using analyses like they presented on 7/15/21:
https://www.sec.gov/Archives/edgar/data/11756...dex992.htm
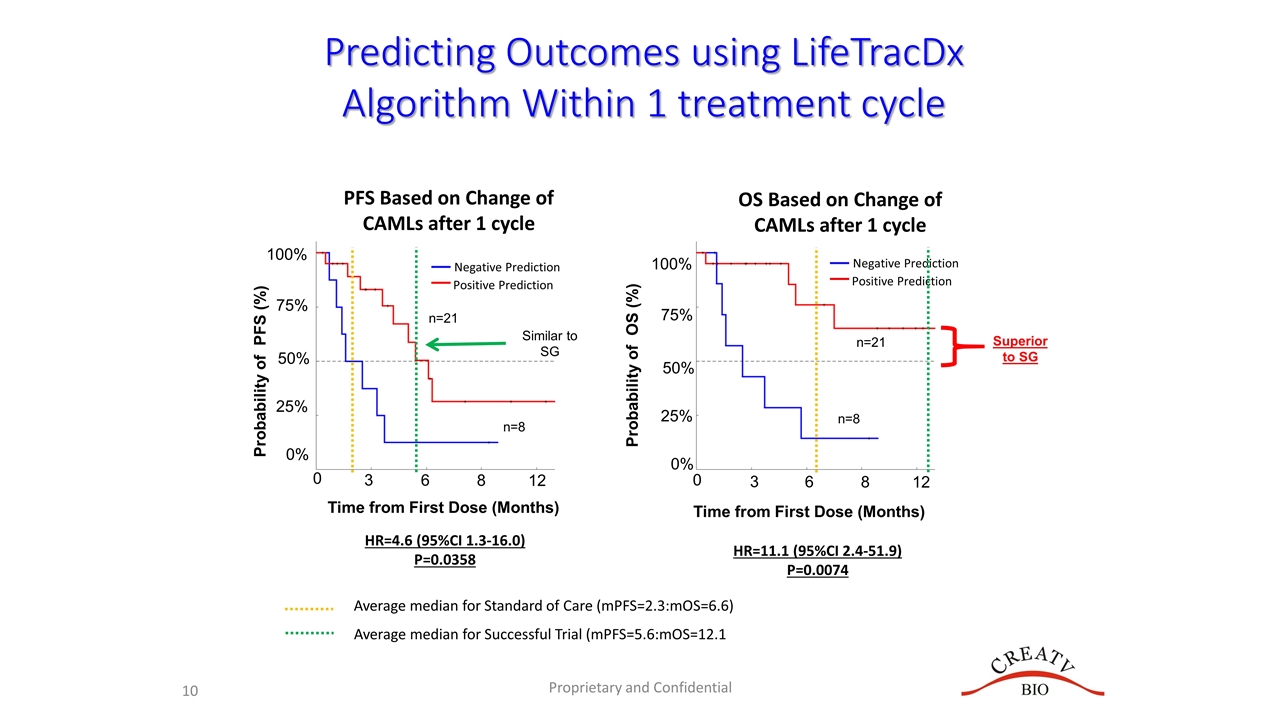
Can't wait to see this extended out further in time. This analysis was from last July and perhaps updated in November.
I'm not sure if one is really able to split up the decreased CTC patients from the rest, as all of them received leronlimab. It is nice to have a biomarker that predicts who will do well after the first dose though.
The 8/25/21 PR says:
Quote:
Decrease in Circulating Tumor Cells (CTC) as reported using the LifeTracDx™ test developed by Creatv MicroTech, Inc., after induction with leronlimab was associated with a 400%-660% increase in mPFS (modified Progression Free Survival)/12-month PFS (Progression Free Survival) and a 570%-980% increase in mOS (modified Overall Survival) /12-month OS (Overall Survival).
[Note it says "modified" Overall Survival, but they probably mean median overall survival.]
Thus, the 980% increase in mOS seems to be an upper bound ("as much as" or "up to" ...) of a confidence interval, using unknown numbers and unknown statistic (does not seem to be simply months of survival, rather some kind of hazard ratio), and apparently using 23 of the 30 combined subjects with low/decreased CTCs.
The 11/3/21 PR one-upped the "980% increase" to "3600%!"
https://www.cytodyn.com/newsroom/press-releas...ysis-of-28
Quote:
75% of the patients who exhibited a lower level of circulating cells after leronlimab (86%) or at baseline (14%) exhibited a 3600% increase in 12-month Overall Survival (“OS”) (p = 0.0004), as compared to a 980% increase in OS reported by the Company on August 25, 2021.
This new analysis removed 2 of the 30 from the analysis to get 28. I forget why they did this.
The 11/8/21 PR gives a hint of where this 980% and 3600% comes from:
https://www.cytodyn.com/newsroom/press-releas...esignation
Quote:
The LifeTracDx™ liquid biopsy algorithm clearly distinguishes responders to leronlimab. 75% of the patients who exhibited lower level of circulating cells after leronlimab or at baseline exhibited statistically significant improvement in mOS (Hazard Ratio (HR): 36.0 (95% CI, 6.2 – 207.6, p=0.0004)) and in mPFS (HR: 5.8 (95% CI, 1.4 – 23.6, p=0.0354)), respectively.
The 36.0 hazard ratio I believe is where he gets the 3600% from (and probably the 980% too). 3600% is the hazard ratio for mOS for those 21 of the 28 combined subjects with low CTCs at baseline (3) or after LL treatment (18), compared to some unspecified historical cohort (or is it compared to the 7 without low CTCs?). 13 of these 28 were from the mTNBC trial (combo with carboplatin), and the rest from compassionate use or the basket trial, at different stages of mTNBC disease.
The confidence intervals given for mOS are apparently on the HR, not months of median overall survival. The hazard ratio gives the ratio of probability of death for those with low CTCs compared to high CTCs (N=21/28 apparently compared to N=7/28, or perhaps compared to some unspecified historical cohort?) using Cox proportional hazards model and Kaplan Meier survival curves, as done below.
I think they are using analyses like they presented on 7/15/21:
https://www.sec.gov/Archives/edgar/data/11756...dex992.htm

Can't wait to see this extended out further in time. This analysis was from last July and perhaps updated in November.
I'm not sure if one is really able to split up the decreased CTC patients from the rest, as all of them received leronlimab. It is nice to have a biomarker that predicts who will do well after the first dose though.
