(Total Views: 699)
Posted On: 07/17/2021 12:02:09 PM
Post# of 157777

Good day to all longs.
The following is an exercise on futility, but an exercise nevertheless ...
I was wondering what the published (preliminary) results of the LH trial mean contextually.
There were 24 symptoms measured and 8 results were published (baseline and 56 days). We know that 6 had no meaningful change and 1 (diarrhea) was worse, therefore 9 more showed improvement as well.
The question is: what can we expect from the whole set? . Do we have a shot at a good p-value for approval once we do our P3?
I analyzed the data we have, that we can consider as the best case scenario. Or, in other words, these are likely the best results. There are two big caveats: I assume the symptoms are not correlated and, we have enough data (large sampling space). None of them are true but, still, I am sharing my data-set & calculations in case somebody finds them useful (I do ):
):
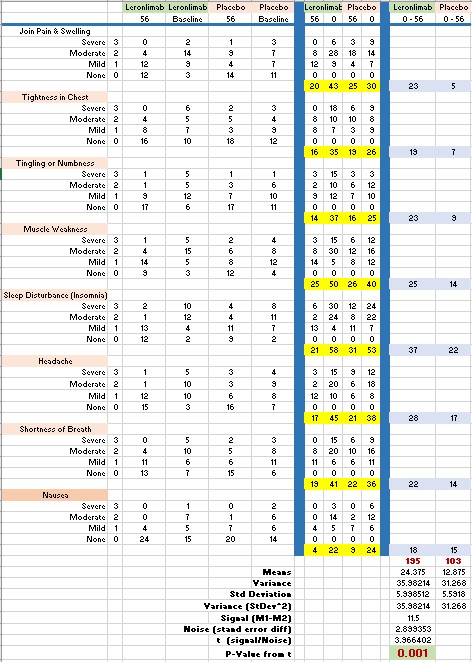
The data is obviously very good with a p-value of 0.001406 This, of course, come from handpicked results. Once we add the not-so-good and no meaningful change and diarrhea this value is going to diminish.
The points: We need to choose carefully our LH trial end-point symptoms for independent non-correlated measures. Also, we definitely have a drug that affects with statistical significance (at least) some of the symptoms of COVID.
imo LH will be a huge market, I think that eventually, the treatment will be a combo of drugs of which LL will/should be a "core" part. We need to design the P3 trial carefully and, as the preliminary statistics show, we have an excellent shot at an approval in this area.
The following is an exercise on futility, but an exercise nevertheless ...
I was wondering what the published (preliminary) results of the LH trial mean contextually.
There were 24 symptoms measured and 8 results were published (baseline and 56 days). We know that 6 had no meaningful change and 1 (diarrhea) was worse, therefore 9 more showed improvement as well.
The question is: what can we expect from the whole set? . Do we have a shot at a good p-value for approval once we do our P3?
I analyzed the data we have, that we can consider as the best case scenario. Or, in other words, these are likely the best results. There are two big caveats: I assume the symptoms are not correlated and, we have enough data (large sampling space). None of them are true but, still, I am sharing my data-set & calculations in case somebody finds them useful (I do

The data is obviously very good with a p-value of 0.001406 This, of course, come from handpicked results. Once we add the not-so-good and no meaningful change and diarrhea this value is going to diminish.
The points: We need to choose carefully our LH trial end-point symptoms for independent non-correlated measures. Also, we definitely have a drug that affects with statistical significance (at least) some of the symptoms of COVID.
imo LH will be a huge market, I think that eventually, the treatment will be a combo of drugs of which LL will/should be a "core" part. We need to design the P3 trial carefully and, as the preliminary statistics show, we have an excellent shot at an approval in this area.
