(Total Views: 594)
Posted On: 05/18/2021 2:28:04 AM
Post# of 158156
FDA Drug Information account on twitter posted 3 times about the 'letter' at about 2 pm (Mountain). So far there are 12 comments.
__________________________
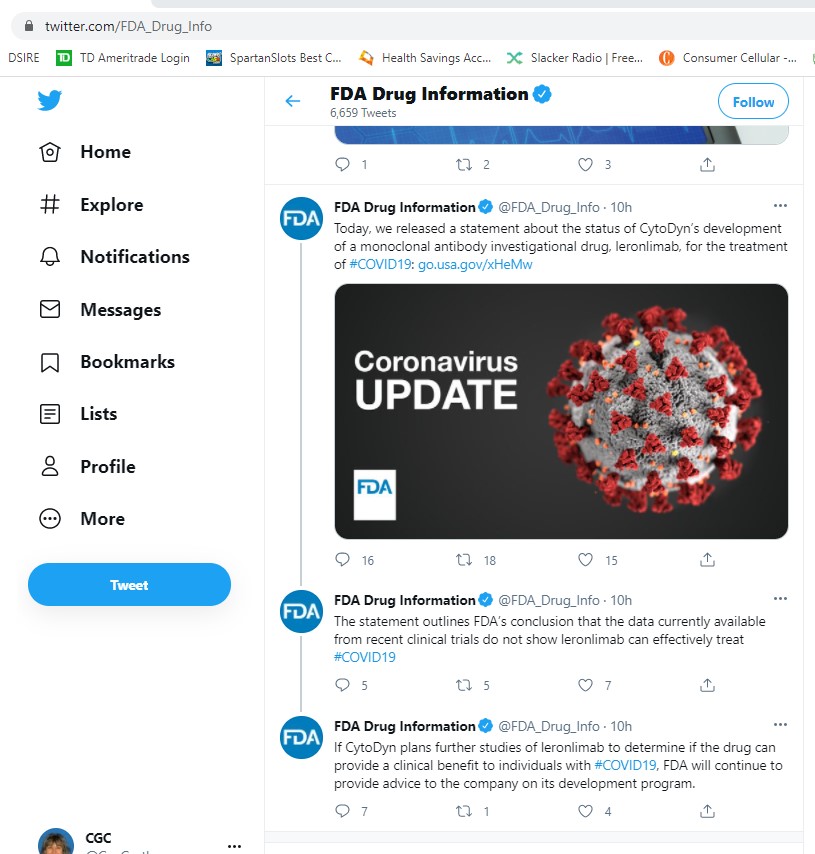
1) Today, we released a statement about the status of CytoDyn’s development of a monoclonal antibody investigational drug, leronlimab, for the treatment of #COVID19: https://go.usa.gov/xHeMw
2) The statement outlines FDA’s conclusion that the data currently available from recent clinical trials do not show leronlimab can effectively treat #COVID19
3) If CytoDyn plans further studies of leronlimab to determine if the drug can provide a clinical benefit to individuals with #COVID19, FDA will continue to provide advice to the company on its development program.
FDA Drug Information Twitter Post
__________________________
1) Today, we released a statement about the status of CytoDyn’s development of a monoclonal antibody investigational drug, leronlimab, for the treatment of #COVID19: https://go.usa.gov/xHeMw
2) The statement outlines FDA’s conclusion that the data currently available from recent clinical trials do not show leronlimab can effectively treat #COVID19
3) If CytoDyn plans further studies of leronlimab to determine if the drug can provide a clinical benefit to individuals with #COVID19, FDA will continue to provide advice to the company on its development program.
FDA Drug Information Twitter Post

