(Total Views: 780)
Posted On: 04/11/2019 12:20:54 PM
Post# of 157546
Leronlimab US Cancer Potential
Independent publication from NCBI about "CCR5 in cancer immunotherapy More than an “attractive” receptor for T cells."
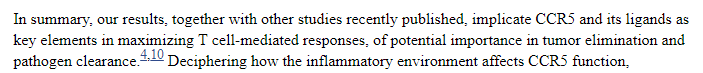
During NobleConXV Conference Presentation RP stated (~15:22 mark):
"CCR5 is necessary and sufficient for the movement of cancer cells to the bone, to the brain, to the lungs and other tissues." My interpretation of these sources is that CCR5 is the 'car' that is required for cancer to metastasize or spread to other parts of the body.
Shortly after RP states that CCR5 is overexpressed in about 50% of breast, prostate, pancreatic, colon and a variety of other types of metastases.
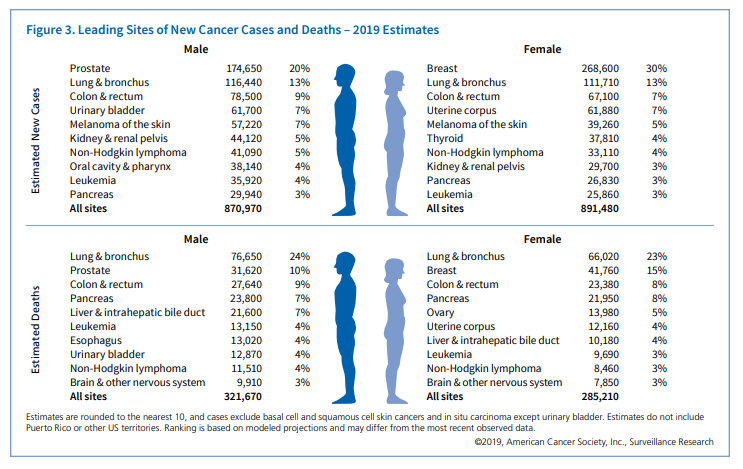
United States 2019 Cancer Facts & Figures
Cancer estimates from cancer.org website for 50% of ONLY these 4 cancers noted by RP during NCXV Presentation (breast, prostate, pancreatic and colon) total 309,000 new patients in 2019. If you add in the other cancers and if they overexpress CCR5 at the same 50% this number more than doubles. Until I can find written proof or RP specifically states other percentages that specific cancers overexpress CCR5, I will leave out of my estimates or calculations for market potential for leronlimab.
Monoclonal antibody HIV treatment w potential FDA approval in 2019 (25 January 2019 Interview with NP)
NP states about the 19:00 mark that leronlimab sales potential will multiply each year. He states the breast cancer market would be 4 times the HER2+ antibody currently approved that generates $15B annually (I beleive it is Trastuzumab), but is only effective treatment due to resistance for 12-18 months. As long as resistance does not build with leronlimab (which thus far it has not shown any resistance levels) the market would multiply annually.....i.e. $60B year 1, $120B year 2, $180B year 3, etc. He also states that if leronlimab proves effective it would work for any cancer metastasis and the numbers would be astronomical.
The price of leronlimab has increased from $24,000 to $70,000 and most recently $120,000 over the past 4-5 months. I am not going to do the calculations, but you get the point of the potential cancer market IF leronlimab is proven in clinical trials. Time to inject and determine whether or not CytoDyn makes 'worldwide news'!!
References:
CCR5 in cancer immunotherapy More than an “attractive” receptor for T cells (1 January 2012)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3376953/
NobleConXV Conference Presentation (28 January 2019)
http://noble.mediasite.com/mediasite/Play/a08...b6137a611d
2019 Cancer Facts & Figures
https://www.cancer.org/content/dam/cancer-org...s-2019.pdf
Monoclonal antibody HIV treatment w potential FDA approval in 2019 Interview (25 January 2019)
https://www.wallstreetreporter.com/2019/01/cy...l-in-2019/
Independent publication from NCBI about "CCR5 in cancer immunotherapy More than an “attractive” receptor for T cells."
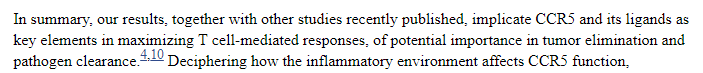
During NobleConXV Conference Presentation RP stated (~15:22 mark):
"CCR5 is necessary and sufficient for the movement of cancer cells to the bone, to the brain, to the lungs and other tissues." My interpretation of these sources is that CCR5 is the 'car' that is required for cancer to metastasize or spread to other parts of the body.
Shortly after RP states that CCR5 is overexpressed in about 50% of breast, prostate, pancreatic, colon and a variety of other types of metastases.
United States 2019 Cancer Facts & Figures
Cancer estimates from cancer.org website for 50% of ONLY these 4 cancers noted by RP during NCXV Presentation (breast, prostate, pancreatic and colon) total 309,000 new patients in 2019. If you add in the other cancers and if they overexpress CCR5 at the same 50% this number more than doubles. Until I can find written proof or RP specifically states other percentages that specific cancers overexpress CCR5, I will leave out of my estimates or calculations for market potential for leronlimab.

Monoclonal antibody HIV treatment w potential FDA approval in 2019 (25 January 2019 Interview with NP)
NP states about the 19:00 mark that leronlimab sales potential will multiply each year. He states the breast cancer market would be 4 times the HER2+ antibody currently approved that generates $15B annually (I beleive it is Trastuzumab), but is only effective treatment due to resistance for 12-18 months. As long as resistance does not build with leronlimab (which thus far it has not shown any resistance levels) the market would multiply annually.....i.e. $60B year 1, $120B year 2, $180B year 3, etc. He also states that if leronlimab proves effective it would work for any cancer metastasis and the numbers would be astronomical.
The price of leronlimab has increased from $24,000 to $70,000 and most recently $120,000 over the past 4-5 months. I am not going to do the calculations, but you get the point of the potential cancer market IF leronlimab is proven in clinical trials. Time to inject and determine whether or not CytoDyn makes 'worldwide news'!!
References:
CCR5 in cancer immunotherapy More than an “attractive” receptor for T cells (1 January 2012)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3376953/
NobleConXV Conference Presentation (28 January 2019)
http://noble.mediasite.com/mediasite/Play/a08...b6137a611d
2019 Cancer Facts & Figures
https://www.cancer.org/content/dam/cancer-org...s-2019.pdf
Monoclonal antibody HIV treatment w potential FDA approval in 2019 Interview (25 January 2019)
https://www.wallstreetreporter.com/2019/01/cy...l-in-2019/
Please do your own due diligence. All my posts and comments are not to be considered investment advice.
