(Total Views: 146)
Posted On: 03/12/2019 8:18:47 AM
Post# of 157927
Yes, not this year, 2020. They are working to get fda approval this year. They are at the BLA stage now with great p3 results. The company should drop upticknews because of their unprofessional behavior last month and now they can’t even get the message correct.
Several different report, surveys, arrive at similar numbers for mdr2 class, over 200k. Below is ome conservation estimate. For 500k yearly revenue at $70k cost per year, they need around 3.5% penetration.
For the market: be first, be early, or be better. Leronlimab falls into the be ’better category’ with its great safety profile and long half-life, offering a change from all the other HIV drugs using daily pills to this, a weekly sc injection. It is also top of its class in this category compared to the other previous approved drugs. I could see 10% penetration happening fairly quick.
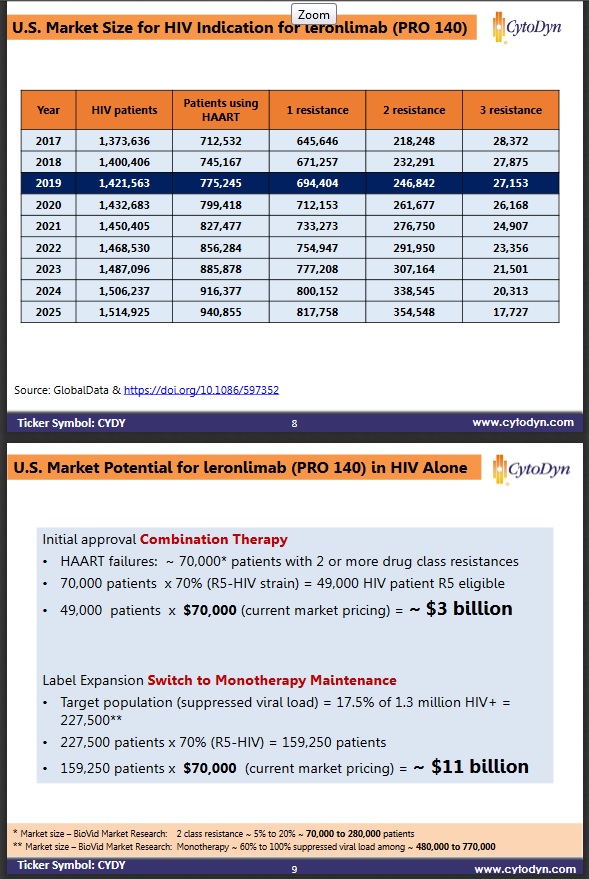
Several different report, surveys, arrive at similar numbers for mdr2 class, over 200k. Below is ome conservation estimate. For 500k yearly revenue at $70k cost per year, they need around 3.5% penetration.
For the market: be first, be early, or be better. Leronlimab falls into the be ’better category’ with its great safety profile and long half-life, offering a change from all the other HIV drugs using daily pills to this, a weekly sc injection. It is also top of its class in this category compared to the other previous approved drugs. I could see 10% penetration happening fairly quick.


