(Total Views: 211)
Posted On: 02/28/2019 8:25:56 AM
Post# of 157708
Next week I would love to see Monotherapy CROI presentation with an announcement early next week of the pivotal trial filing
They said the 1st part of the BLA is ready to go. They listed the rolling BLA starting in February on a presentation this month, which ends today. NP said on Monday, they will fill for the pivotal trial after that first submission from combo. If there was ever a perfect time to announce it, it would be next week. They would be the talk of the conference I feel.
Of course this assumes the meeting with the FDA about mono, didn't have some more conditions we are not aware, like a little more data with the 700mg group first.
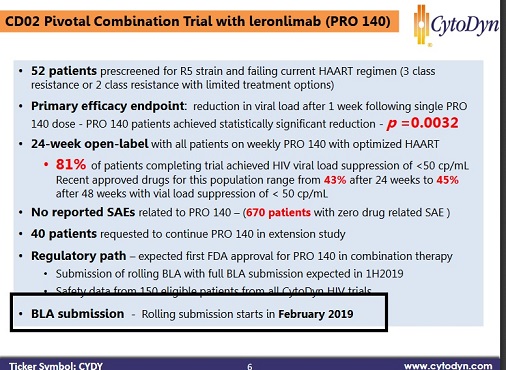
They said the 1st part of the BLA is ready to go. They listed the rolling BLA starting in February on a presentation this month, which ends today. NP said on Monday, they will fill for the pivotal trial after that first submission from combo. If there was ever a perfect time to announce it, it would be next week. They would be the talk of the conference I feel.
Of course this assumes the meeting with the FDA about mono, didn't have some more conditions we are not aware, like a little more data with the 700mg group first.

Quote:
14:30 submission quickly as possible in
14:33 regards to mono therapy trial we are to
14:36 present at CROI on march 4th our
14:39 abstract was accepted and we are excited
14:41 to go back to croy and say that our last
14:45 trial that we presented at CROI that had
14:48 only 40% responders rate now is change
14:54 and there with a seven hundred milligram
14:56 we have much better data and 525
14:59 milligram
15:00 so that we will follow through but we
15:03 are working on our pivotal trial and
15:05 once we submit our first part of our BLA
15:07 which is already complete
15:09 one third of a non-clinical section then
15:12 we will follow with our protocol for a
15:15 mono therapy pivotal trial

