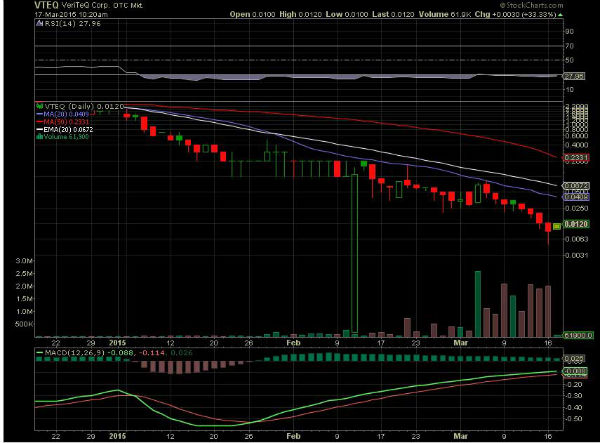
Posted On: 03/17/2015 10:39:23 AM
Post# of 145495
$VTEQ ~ an FDA cleared micro-transponder technology (Q Inside Safety Technology(TM)) that acts as an electronic serial number enabling physicians and patients to access a secure, online database to retrieve implant-specific data, such as serial number, manufacturer name, date of manufacture, lot number, volume, size, and other data from the device manufacturer. Q Inside Safety Technology(TM) may also provide an extra level of protection to the patient in the event of a recall or other safety event.
http://finance.yahoo.com/news/largest-provide...55051.html
DELRAY BEACH, Fla., March 12, 2015 (GLOBE NEWSWIRE) -- VeriTeQ Corporation ("VeriTeQ" (OTC Markets:VTEQ), a provider of implantable medical device identification and radiation dose measurement technologies, and Establishment Labs, S.A. ("EL"
(OTC Markets:VTEQ), a provider of implantable medical device identification and radiation dose measurement technologies, and Establishment Labs, S.A. ("EL" announced today that The Hospital Group, the largest provider of cosmetic surgery in the United Kingdom ("UK"
announced today that The Hospital Group, the largest provider of cosmetic surgery in the United Kingdom ("UK" , now offers EL's Motiva Implant Matrix(R) breast implants with VeriTeQ's Q Inside Safety Technology(TM) to patients choosing to undergo breast augmentation. The Hospital Group operates a network of more than 20 clinics around the UK and Ireland.
, now offers EL's Motiva Implant Matrix(R) breast implants with VeriTeQ's Q Inside Safety Technology(TM) to patients choosing to undergo breast augmentation. The Hospital Group operates a network of more than 20 clinics around the UK and Ireland.
http://finance.yahoo.com/news/largest-provide...55051.html
DELRAY BEACH, Fla., March 12, 2015 (GLOBE NEWSWIRE) -- VeriTeQ Corporation ("VeriTeQ"

