Posted On: 12/10/2014 1:33:20 PM
Post# of 103182
Re: lookn4wins #44597
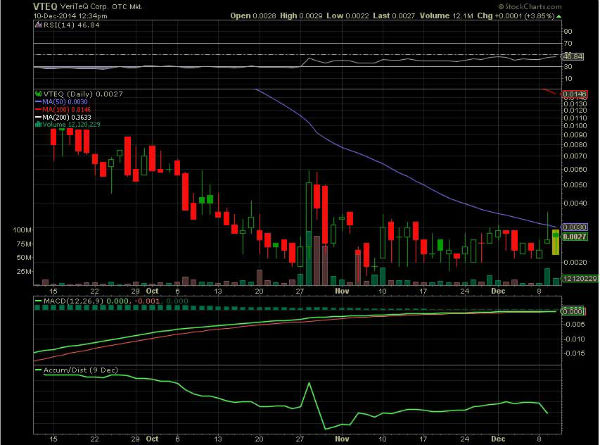
VTEQ ~ Trying to break above 50 Day SMA "FDA CLEARED"
VeriTeQ's FDA cleared Q Inside Safety Technology(TM) acts as an electronic serial number in breast implants and other implantable and reusable medical devices to provide physicians and patients access to secure online databases to retrieve device-specific data, such as serial number, manufacturer name, date of manufacture, lot number, volume, size, and other data from the medical device manufacturer. Q Inside Safety Technology(TM) may also provide an extra level of protection to the patient in the event of a recall or other safety event.
VeriTeQ's FDA cleared Q Inside Safety Technology(TM) acts as an electronic serial number in breast implants and other implantable and reusable medical devices to provide physicians and patients access to secure online databases to retrieve device-specific data, such as serial number, manufacturer name, date of manufacture, lot number, volume, size, and other data from the medical device manufacturer. Q Inside Safety Technology(TM) may also provide an extra level of protection to the patient in the event of a recall or other safety event.

